|
Sulfonyl
In organosulfur chemistry, a sulfonyl group is either a functional group found primarily in sulfones, or a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Group Sulfonyl groups can be written as having the general formula , where there are two double bonds between the sulfur and oxygen. Sulfonyl groups can be reduced to the sulfide with diisobutylaluminium hydride (DIBALH). Lithium aluminium hydride () reduces some but not all sulfones to sulfides. In inorganic chemistry, when the group is not connected to any carbon atoms, it is referred to as sulfuryl. Examples of sulfonyl group substituents The names of sulfonyl groups typically end in -syl, such as: : See also * Sulfonyl halide * Sulfonamide * Sulfonate * Methylsulfonylmethane Dimethyl sulfone (DMSO2) is an organosulfur compound with the formula . It is also known by several other names including methyl sulfone and (especially in alternative medicine) methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonyl Halide
In chemistry, a sulfonyl halide consists of a sulfonyl () group singly bonded to a halogen atom. They have the general formula , where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series. Sulfonyl halides have tetrahedral sulfur centres attached to two oxygen atoms, an organic radical, and a halide. In a representative example, methanesulfonyl chloride, the S=O, S−C, and S−Cl bond distances are respectively 142.4, 176.3, and 204.6 pm. Sulfonyl chlorides Sulfonic acid chlorides, or sulfonyl chlorides, are a sulfonyl halide with the general formula . Production Arylsulfonyl chlorides are made industrially in a two-step, one-pot reaction from an arene (in this case, benzene) and chlorosulfuric acid: : : The intermediate benzenesulfonic acid can be chlorinated with thionyl chloride as well. Benz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylsulfonylmethane
Dimethyl sulfone (DMSO2) is an organosulfur compound with the formula . It is also known by several other names including methyl sulfone and (especially in alternative medicine) methylsulfonylmethane (MSM). This colorless solid features the sulfonyl functional group and is the simplest of the sulfones. It is relatively inert chemically and is able to resist decomposition at elevated temperatures. It occurs naturally in some primitive plants, is present in small amounts in many foods and beverages, and is marketed (under the MSM name) as a dietary supplement. It is sometimes used as a cutting agent for illicitly manufactured methamphetamine. It is also commonly found in the atmosphere above marine areas, where it is used as a carbon source by the airborne bacteria '' Afipia''. Oxidation of dimethyl sulfoxide produces the sulfone, both under laboratory conditions and metabolically. Use as a solvent Because of its polarity and thermal stability, molten DMSO2 has been used industria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesyl Chloride
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula . Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group –, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).Valerie Vaillancourt, Michele M. Cudahy, Matthew M. Kreilein and Danielle L. Jacobs "Methanesulfonyl Chloride" in E-EROS Encyclopedia for Reagents in Organic Synthesis Preparation It is produced by the reaction of methane and sulfuryl chloride in a radical reaction: : Another method of production entails chlorination of methanesulfonic acid with thionyl chloride or phosgene: : : Reactions Methanesulfonyl chloride is a precursor to many compounds because it is highly re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonyl Groups
In organosulfur chemistry, a sulfonyl group is either a functional group found primarily in sulfones, or a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Group Sulfonyl groups can be written as having the general formula , where there are two double bonds between the sulfur and oxygen. Sulfonyl groups can be reduced to the sulfide with diisobutylaluminium hydride (DIBALH). Lithium aluminium hydride () reduces some but not all sulfones to sulfides. In inorganic chemistry, when the group is not connected to any carbon atoms, it is referred to as sulfuryl. Examples of sulfonyl group substituents The names of sulfonyl groups typically end in -syl, such as: : See also * Sulfonyl halide * Sulfonamide * Sulfonate * Methylsulfonylmethane Dimethyl sulfone (DMSO2) is an organosulfur compound with the formula . It is also known by several other names including methyl sulfone and (especially in alternative medicine) methylsu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosyl Chloride
4-Toluenesulfonyl chloride (''p''-toluenesulfonyl chloride, toluene-''p''-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or TosCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group. Uses As typical for Sulfonyl halides, TsCl converts alcohols (abbreviated ROH) into the corresponding toluenesulfonate esters, or tosyl derivatives ("tosylates"): : CH3C6H4SO2Cl + ROH → CH3C6H4SO2OR + HCl Tosylates can be cleaved with lithium aluminium hydride: : 4 CH3C6H4SO2OR + LiAlH4 → LiAl(O3SC6H4CH3)4 + 4 RH Thus, tosylation followed by reduction allows for removal of a hydroxyl group. Likewise, TsCl is used to prepare sulfonamides from amines: :CH3C6H4SO2Cl + R2NH → CH3C6H4SO2NR2 + HCl The resulting sulfonamides are non-basic and, when derived from primary amines, are even acidic. TsCl reacts with hydrazine to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudoelement Symbol
The skeletal formula, line-angle formula, bond-line formula or shorthand formula of an organic compound is a type of minimalist structural formula representing a molecule's atoms, bonds and some details of its geometry. The lines in a skeletal formula represent bonds between carbon atoms, unless labelled with another element. Labels are optional for carbon atoms, and the hydrogen atoms attached to them. An early form of this representation was first developed by organic chemist August Kekulé, while the modern form is closely related to and influenced by the Lewis structure of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures or Lewis–Kekulé structures. Skeletal formulas have become ubiquitous in organic chemistry, partly because they are relatively quick and simple to draw, and also because the curved arrow notation used for discussions of reaction mechanisms and electron delocalization can be readily superimposed. Several oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents. Synthesis and reactions By oxidation of thioethers and sulfoxides Sulfones are typically prepared by organic oxidation of thioethers, often referred to as sulfides. Sulfoxides are intermediates in this route. For example, dimethyl sulfide oxidizes to dimethyl sulfoxide and then to dimethyl sulfone. From SO2 : Sulfur dioxide is a convenient and widely used source of the sulfonyl functional group. Specifically, Sulfur dioxide participates in cycloaddition reactions with dienes. The industrially useful solvent sulfolane is prepared by addition of sulfur dioxide to buta-1,3-diene followed by hydrogenation of the resulting sulfolene. From sulfonyl and sulfuryl halides Sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
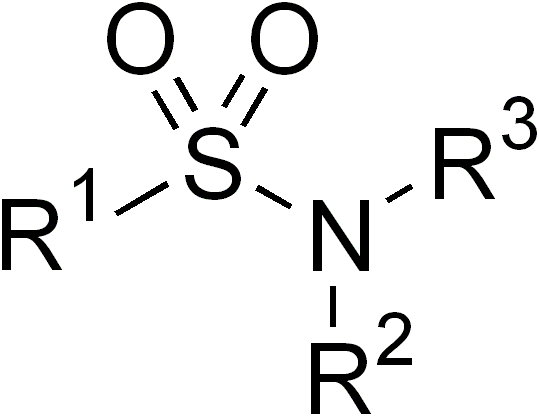
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: : In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. In a related process, carboxyli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl group, , joined to a sulfonyl group, , with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group. The tosyl terminology was proposed by German chemists Kurt Hess and Robert Pfleger in 1933 on the pattern of trityl and adopted in English starting from 1934. The toluenesulfonate (or tosylate) group refers to the (–OTs) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |