|
Stibine
Stibine (IUPAC name: stibane) is a chemical compound with the formula Sb H3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). The smell of this compound from usual sources (like from reduction of antimony compounds) is reminiscent of arsine, i.e. garlic-like. Preparation SbH3 is generally prepared by the reaction of Sb3+ sources with H− equivalents: :2 Sb2O3 + 3 LiAlH4 → 4 SbH3 + 1.5 Li2O + 1.5 Al2O3 :4 SbCl3 + 3 NaBH4 → 4 SbH3 + 3 NaCl + 3 BCl3 Alternatively, sources of Sb3− react with protonic reagents (even water) to also produce this unstable gas: :Na3Sb + 3 H2O → SbH3 + 3 NaOH Properties The chemical properties of SbH3 resemble those for AsH3. Typical for a heavy hydride (e.g. AsH3, H2Te, SnH4), SbH3 is unstable with respect to its elements. The gas decomposes slowly at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylstibine
Triphenylstibine is the chemical compound with the formula Sb(C6H5)3, which is often abbreviated SbPh3, This colourless solid is a common organoantimony(III) compound. It serves as a ligand in coordination chemistry and as a reagent in organic synthesis. Like the related molecules triphenylamine, triphenylphosphine and triphenylarsine, SbPh3 is pyramidal with a propeller-like arrangement of the phenyl groups. The Sb-C distances average 2.14-2.17 Å and the C-Sb-C angles are 95°. Synthesis and reactions Triphenylstibine was first reported in 1886, being prepared from antimony trichloride and chlorobenzene: :6 Na + 3 C6H5Cl + SbCl3 → (C6H5)3Sb + 6 NaCl In an alternative method, phenylmagnesium bromide is treated with SbCl3. Upon treatment with antimony trichloride, triphenylstibine undergoes a redistribution reaction: : Stiboranes can be synthesised from triphenylstibine by halogenation: : As confirmed by X-ray crystallography X-ray crystallography is the experiment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pnictogen Hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, bismuth, and moscovium) covalently bonded to hydrogen. Pnictogen trihydrides The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements. Some properties of the pnictogen trihydrides follow: These gases have no smell in pure form, instead gaining it when in contact with air. Ammonia has an infamous, intense odour resembling urine and/or fish, commonly the result of the decomposition of urea. Phosphine smells like fish or garlic, and stibine like rotten eggs, similar to hydrogen sulfide a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−''x''R''x'', where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties In its standard state arsine is a colorless, denser-than-air gas that is slightly soluble in water (2% at 20 °C) and in many organic solvents as well. Arsine itself is odorless, but it oxidizes in air and this creates a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230 °C, decomp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name Kohl (cosmetics), kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio. China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are Roasting (metallurgy), roasting followed by carbothermic reaction, reduction with carbon, or direct reduction of stibnite with iron. The most common applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, Bullet, bullets, and plain bearings. It improves the rigidity of lead-alloy pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
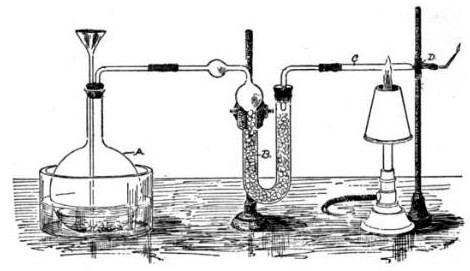
Marsh Test
The Marsh test is a highly sensitive method in the detection of arsenic, especially useful in the field of forensic toxicology when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836. The method continued to be used, with improvements, in forensic toxicology until the 1970s. Arsenic, in the form of white arsenic trioxide , was a highly favored poison, being odourless, easily incorporated into food and drink, and before the advent of the Marsh test, untraceable in the body. In France, it came to be known as ' ("inheritance powder"). For the untrained, arsenic poisoning will have symptoms similar to cholera. Precursor methods The first breakthrough in the detection of arsenic poisoning was in 1775 when Carl Wilhelm Scheele discovered a way to change arsenic trioxide to garlic-smelling arsine gas (), by treating it with nitric acid () and combining it with zinc: : In 1787, German physician (1739-1805) discovered that if arseni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
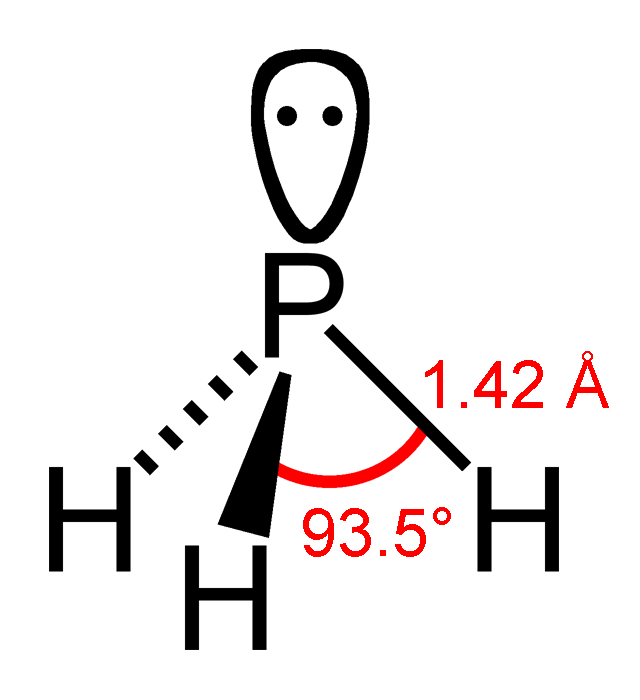
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuthine
Bismuthine (IUPAC name: bismuthane) is the chemical compound with the formula Bi H3. As the heaviest analogue of ammonia (a pnictogen hydride), BiH3 is unstable, decomposing to bismuth metal well below 0 °C. This compound adopts the expected pyramidal structure with H–Bi–H angles of around 90°. The term ''bismuthine'' may also refer to a member of the family of organobismuth(III) species having the general formula , where R is an organic substituent. For example, Bi(CH3)3 is ''trimethylbismuthine''. Preparation and properties BiH3 is prepared by the redistribution of methylbismuthine (BiH2Me):Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001.. :3 BiH2Me → 2 BiH3 + BiMe3 The required BiH2Me, which is also thermally unstable, is generated by reduction of methylbismuth dichloride, BiCl2Me with LiAlH4. As suggested by the behavior of SbH3, BiH3 is unstable and decomposes to its constituent elements according to the following equat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |