|
Standard Treatment
The standard treatment, also known as the standard of care, is the medical treatment that is normally provided to people with a given condition. In many scientific studies, the control group receives the standard treatment rather than a placebo while a treatment group receives the experimental treatment. After the clinical trial, researchers compare the outcomes of the two groups to see if the experimental treatment is better than, as good as or not as beneficial as the standard treatment. Active (positive) concurrent control In an active control or positive control trial, subjects are randomly assigned to the test treatment or to an active control treatment. Such clinical trials are usually double-blind, but this is not always possible; many oncology trials, for example, are considered difficult or impossible to blind because of different regimens, different routes of administration, and different toxicities. Active control trials can have two distinct objectives with respect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapy
A therapy or medical treatment is the attempted remediation of a health problem, usually following a medical diagnosis. Both words, ''treatment'' and ''therapy'', are often abbreviated tx, Tx, or Tx. As a rule, each therapy has indications and contraindications. There are many different types of therapy. Not all therapies are effective. Many therapies can produce unwanted adverse effects. ''Treatment'' and ''therapy'' are often synonymous, especially in the usage of health professionals. However, in the context of mental health, the term ''therapy'' may refer specifically to psychotherapy. Semantic field The words ''care'', ''therapy'', ''treatment'', and ''intervention'' overlap in a semantic field, and thus they can be synonymous depending on context. Moving rightward through that order, the connotative level of holism decreases and the level of specificity (to concrete instances) increases. Thus, in health-care contexts (where its senses are always nonc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disease
A disease is a particular abnormal condition that adversely affects the structure or function (biology), function of all or part of an organism and is not immediately due to any external injury. Diseases are often known to be medical conditions that are associated with specific signs and symptoms. A disease may be caused by external factors such as pathogens or by internal dysfunctions. For example, internal dysfunctions of the immune system can produce a variety of different diseases, including various forms of immunodeficiency, hypersensitivity, allergy, allergies, and autoimmune disorders. In humans, ''disease'' is often used more broadly to refer to any condition that causes pain, Abnormality (behavior), dysfunction, distress (medicine), distress, social problems, or death to the person affected, or similar problems for those in contact with the person. In this broader sense, it sometimes includes injury in humans, injuries, disability, disabilities, Disorder (medicine) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Control Group
In the design of experiments, hypotheses are applied to experimental units in a treatment group. In comparative experiments, members of a control group receive a standard treatment, a placebo, or no treatment at all. There may be more than one treatment group, more than one control group, or both. A placebo control group can be used to support a double-blind study, in which some subjects are given an ineffective treatment (in medical studies typically a sugar pill) to minimize differences in the experiences of subjects in the different groups; this is done in a way that ensures no participant in the experiment (subject or experimenter) knows to which group each subject belongs. In such cases, a third, non-treatment control group can be used to measure the placebo effect directly, as the difference between the responses of placebo subjects and untreated subjects, perhaps paired by age group or other factors (such as being twins). For the conclusions drawn from the results of an ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) can be roughly defined as a sham medical treatment. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures. Placebos are used in randomized clinical trials to test the efficacy of medical treatments. In a placebo-controlled trial, any change in the control group is known as the ''placebo response'', and the difference between this and the result of no treatment is the ''placebo effect''. Placebos in clinical trials should ideally be indistinguishable from so-called verum treatments under investigation, except for the latter's particular hypothesized medicinal effect. This is to shield test participants (with their consent) from knowing who is getting the placebo and who is getting the treatment under test, as patients' and clinicians' expectations of efficacy can influence results. The idea of a placebo effect was discussed in 18th century psychology, but became more prominent in the 20th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double-blind
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, Observer-expectancy effect, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints. During the course of an experiment, a participant becomes #Unblinding, unblinded if they deduce or otherwise obtain information that has been masked to them. For example, a patient w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
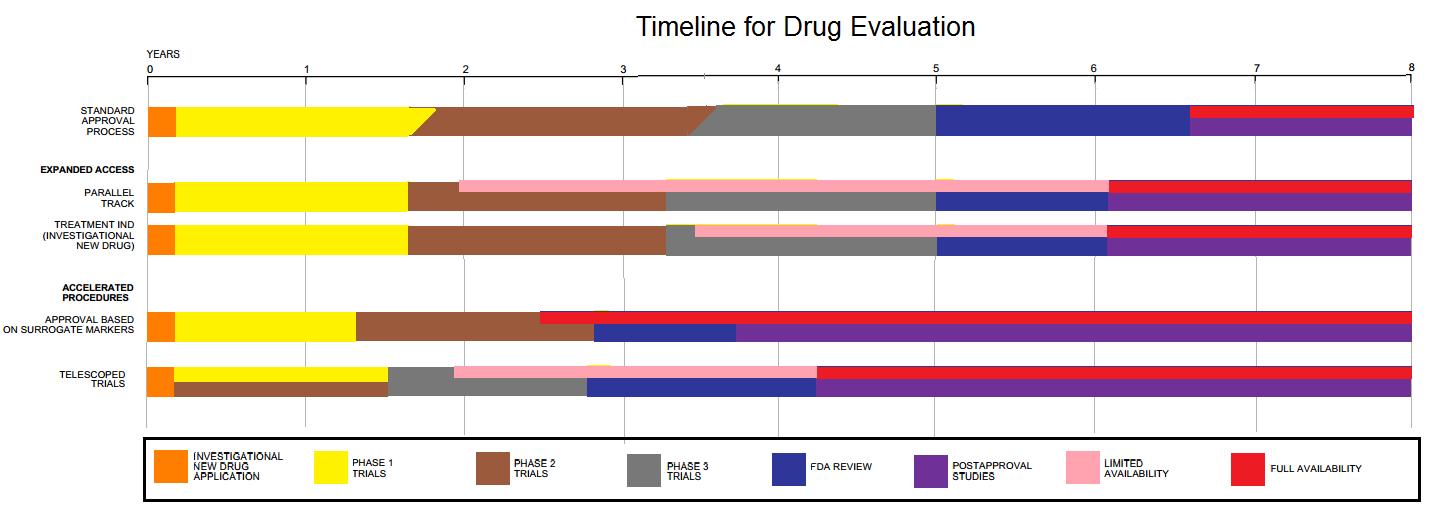
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. These h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Drug Application
The Food and Drug Administration's (FDA) New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of initial drug candidates proceed through the entire multi-year process of drug development, concluding with an approved NDA, if successful. The goals of the NDA are to provide enough information to permit FDA reviewers to establish the complete history of the candidate drug. Among facts needed for the application are: * Patent and manufacturing information * Drug safety and specific effectiveness for its proposed use(s) when used as directed * Reports on the design, compliance, and conclusions of completed clinical trials by the Institutional Review Board * Drug susceptibility to substance abuse, abuse * Proposed labeling (package insert) and directions for use Exceptions to this process include voter driven initiatives for Medical cannabis, medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration (United States)
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs but involves other factors like regulating lasers, cellular phones, and condoms. In addition, the FDA takes control of disea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an initiative that brings together regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceutical product development and registration. The mission of the ICH is to promote public health by achieving greater harmonisation through the development of technical guidelines and requirements for pharmaceutical product registration. Harmonisation leads to a more rational use of human, animal and other resources, the elimination of unnecessary delay in the global development, and availability of new medicines while maintaining safeguards on quality, safety, efficacy, and regulatory obligations to protect public health. Junod notes in her 2005 treatise on clinical drug trials that " ove all, the ICH has succeeded in aligning clinical trial requirements." History In the 1980s, the European Union began harmonising regulatory requir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness (efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. These research procedures are designed for the prevention, treatment, diagnosis or understanding of disease symptoms. Clinical research is different from clinical practice: in clinical practice, established treatments are used to improve the condition of a person, while in clinical research, evidence is collected under rigorous study conditions on groups of people to determine the efficacy and safety of a treatment. Description The term "clinical research" refers to the entire process of studying and writing about a drug, a medical device or a form of treatment, which includes conducting interventional studies (clinical trials) or observational studies on human participants. Clinical research can cover any medical method or product from it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |