|
New Drug Application
The Food and Drug Administration's (FDA) New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of initial drug candidates proceed through the entire multi-year process of drug development, concluding with an approved NDA, if successful. The goals of the NDA are to provide enough information to permit FDA reviewers to establish the complete history of the candidate drug. Among facts needed for the application are: * Patent and manufacturing information * Drug safety and specific effectiveness for its proposed use(s) when used as directed * Reports on the design, compliance, and conclusions of completed clinical trials by the Institutional Review Board * Drug susceptibility to substance abuse, abuse * Proposed labeling (package insert) and directions for use Exceptions to this process include voter driven initiatives for Medical cannabis, medic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complete Response Letter
In United States pharmaceutical regulatory practice, a Complete Response Letter (CRL), or more rarely, a 314.110 letter, is a regulatory action by the Food and Drug Administration in response to a New Drug Application, Amended New Drug Application or Biologics License Application, indicating that the application will not be approved in its present form. CRLs replaced approvable letters in 2018. Background Under the Prescription Drug User Fee Act, the Food and Drug Administration has a limited timespan (known as the PDUFA date) to decide a New Drug Application, Abbreviated New Drug Application or Biologics License Application. The FDA may either approve the application or issue a Complete Response Letter. Grounds behind issuing a CRL may include labeling issues, current Good Manufacturing Practice concerns or concerns about the safety or effectiveness of the drug. A sponsor receiving CRL may withdraw the application, request a hearing or resubmit the application. Because heari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Animal Drug Application
A New Animal Drug Application is an American legal terminology, defined in 21 CFR ¶514, after the definition in ¶510 of the term New Animal Drug. It is utilized by the FDA. A new animal drug is defined, in part, as any drug intended for use in animals other than man, including any drug intended for use in animal feed but not including the animal feed, the composition of which is such that the drug is not generally recognized as safe and effective for the use under the conditions prescribed, recommended, or suggested in the labeling of the drug. It was mandated by the Federal Food, Drug, and Cosmetic Act, as modified by Food and Drug Administration Amendments Act of 2007 on 27 September 2007, and is the analogue of the New Drug Application The Food and Drug Administration's (FDA) New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of init ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Center For Veterinary Medicine
The Center for Veterinary Medicine (CVM) is a branch of the U.S. Food and Drug Administration (FDA) that regulates the manufacture and distribution of food, food additives, and drugs that will be given to animals. These include animals from which human foods are derived, as well as food additives and drugs for pets or companion animals. CVM is responsible for regulating drugs, devices, and food additives given to, or used on, over one hundred million companion animals, plus millions of poultry, cattle, swine, and minor animal species. Minor animal species include animals other than cattle, swine, chickens, turkeys, horses, dogs, and cats. CVM monitors the safety of animal foods and medications. Much of the center's work focuses on animal medications used in food animals to ensure that significant drug residues are not present in the meat or other products from these animals. CVM does not regulate vaccines for animals; these are handled by the United States Department of Agric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyaluronidase
Hyaluronidases are a family of enzymes that catalyse the degradation of hyaluronic acid. Karl Meyer classified these enzymes in 1971, into three distinct groups, a scheme based on the enzyme reaction products. The three main types of hyaluronidases are two classes of eukaryotic endoglycosidase hydrolases and a prokaryotic lyase-type of glycosidase. In humans, there are five functional hyaluronidases: HYAL1, HYAL2, HYAL3, HYAL4 and HYAL5 (also known as SPAM1 or PH-20); plus a pseudogene, HYAL6 (also known as HYALP1). The genes for HYAL1-3 are clustered in chromosome 3, while HYAL4-6 are clustered in chromosome 7. HYAL1 and HYAL2 are the major hyaluronidases in most tissues. GPI-anchored HYAL2 is responsible for cleaving high-molecular weight hyaluronic acid, which is mostly bound to the CD44 receptor. The resulting hyaluronic acid fragments of variable size are then further hydrolyzed by HYAL1 after being internalized into endo-lysosomes; this generates hyaluronic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcitonin
Calcitonin is a 32 amino acid peptide hormone secreted by parafollicular cells (also known as C cells) of the thyroid (or endostyle) in humans and other chordates in the ultimopharyngeal body. It acts to reduce blood calcium (Ca2+), opposing the effects of parathyroid hormone (PTH). Its importance in humans has not been as well established as its importance in other animals, as its function is usually not significant in the regulation of normal Calcium metabolism, calcium homeostasis. It belongs to the calcitonin-like protein family. Historically calcitonin has also been called thyrocalcitonin. Biosynthesis and regulation Calcitonin is formed by the proteolytic cleavage of a larger prepropeptide, which is the product of the CALC1 gene (). It is functionally an antagonist with PTH and Vitamin D3. The CALC1 gene belongs to a superfamily of related protein hormone precursors including islet amyloid precursor protein, calcitonin gene-related peptide, and the precursor of adrenomedul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body. It is also used as a Glucagon (medication), medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the ''GCG'' gene. The pancreas releases glucagon when the amount of glucose in the bloodstream is too low. Glucagon causes the liver to engage in glycogenolysis: converting stored glycogen into glucose, which is released into the bloodstream. High blood-glucose levels, on the other hand, stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels stable. Glucagon increases energy expenditure and is elevated under conditions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
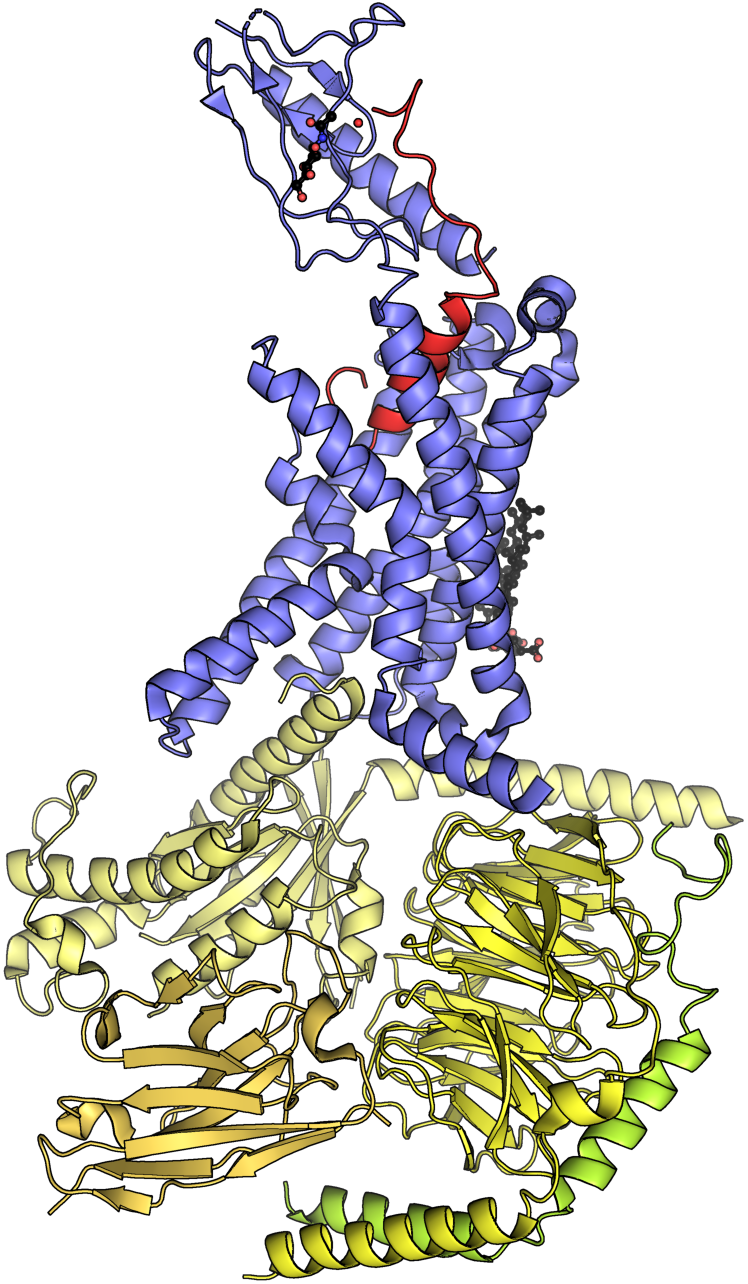
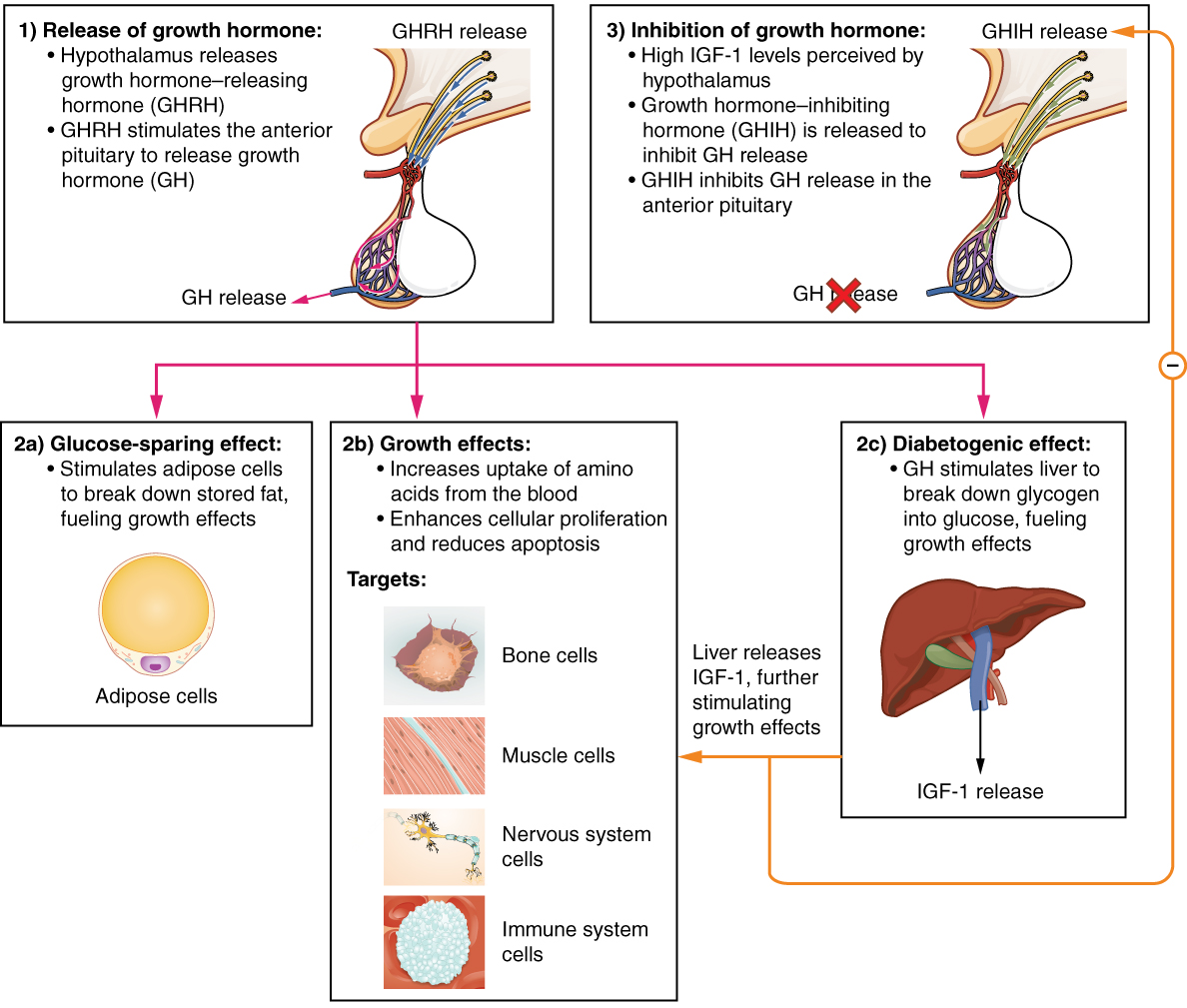
Growth Hormone
Growth hormone (GH) or somatotropin, also known as human growth hormone (hGH or HGH) in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in human development. GH also stimulates production of insulin-like growth factor 1 (IGF-1) and increases the concentration of glucose and free fatty acids. It is a type of mitogen which is specific only to the receptors on certain types of cells. GH is a 191-amino acid, single-chain polypeptide that is synthesized, stored and secreted by somatotropic cells within the lateral wings of the anterior pituitary gland. A recombinant form of HGH called somatropin ( INN) is used as a prescription drug to treat children's growth disorders and adult growth hormone deficiency. In the United States, it is only available legally from pharmacies by prescription from a licensed health care provider. In recent years in the United States, some health ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (''INS)'' gene. It is the main Anabolism, anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats, and protein by promoting the absorption of glucose from the blood into cells of the liver, fat cell, fat, and skeletal muscles. In these tissues the absorbed glucose is converted into either glycogen, via glycogenesis, or Fatty acid metabolism#Glycolytic end products are used in the conversion of carbohydrates into fatty acids, fats (triglycerides), via lipogenesis; in the liver, glucose is converted into both. Glucose production and secretion by the liver are strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is thus an anabolic hormone, promoting the conversion of small molecules in the blood into large ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abbreviated New Drug Application
An Abbreviated New Drug Application (ANDA) is an application for a U.S. generic drug approval for an existing licensed medication or approved drug. The ANDA is submitted to FDA's Center for Drug Evaluation and Research, Office of Generic Drugs, which provides for the review and ultimate approval of a generic drug product. Once approved, an applicant may manufacture and market the generic drug product to provide a safe, effective, low cost alternative to the American public. Electronic submissions of ANDAs have grown by 70% since November 2008. The Section IV challenge has been credited with suppressing new drug innovation. A generic drug product is one that is comparable to a patented drug product in dosage form, strength, route of administration, quality, performance characteristics and intended use. All approved products, both innovator and generic, are listed in FDA's Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book). Generic drug applications are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance compared to their performance at the time when they were patented drugs. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |