|
Standard Atomic Weight
The standard atomic weight of a chemical element (symbol ''A''r°(E) for element "E") is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotope's abundance on Earth. For example, isotope 63Cu (''A''r = 62.929) constitutes 69% of the copper on Earth, the rest being 65Cu (''A''r = 64.927), so :A_\text\text(_\text\text)=0.69\times62.929+0.31\times64.927=63.55. Relative isotopic mass is dimensionless, and so is the weighted average. It can be converted into a measure of mass (with dimension ) by multiplying it with the atomic mass constant dalton. Among various variants of the notion of atomic weight (''A''r, also known as ''relative atomic mass'') used by scientists, the standard atomic weight is the most common and practical. The standard atomic weight of each chemical element is determined and published by the Commission on Isotopic Abundances and Atomic Weights (CIAAW) of the International Union of Pure and App ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons, due to mass defect (explained by mass-energy equivalence: ). Atomic mass is often measured in dalton (Da) or unified atomic mass unit (u). One dalton is equal to the mass of a carbon-12 atom in its natural state, given by the atomic mass constant , where is the atomic mass of carbon-12. Thus, the numerical value of the atomic mass of a nuclide when expressed in daltons is close to its mass number. The relative isotopic mass (see section below) can be obtained by dividing the atomic mass of an isotope by the atomic mass constant , yielding a dimensionless value. Thus, the atomic mass of a carbon-12 atom is b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth's Atmosphere
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weather features such as clouds and hazes), all retained by Earth's gravity. The atmosphere serves as a protective buffer between the Earth's surface and outer space, shields the surface from most meteoroids and ultraviolet solar radiation, keeps it warm and reduces diurnal temperature variation (temperature extremes between day and night) through heat retention (greenhouse effect), redistributes heat and moisture among different regions via air currents, and provides the chemical and climate conditions allowing life to exist and evolve on Earth. By mole fraction (i.e., by quantity of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other trace gases (see Composition below ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astrophysical Journal
''The Astrophysical Journal'' (''ApJ'') is a peer-reviewed scientific journal of astrophysics and astronomy, established in 1895 by American astronomers George Ellery Hale and James Edward Keeler. The journal discontinued its print edition and became an electronic-only journal in 2015. Since 1953, ''The Astrophysical Journal Supplement Series'' (''ApJS'') has been published in conjunction with ''The Astrophysical Journal'', with generally longer articles to supplement the material in the journal. It publishes six volumes per year, with two 280-page issues per volume. ''The Astrophysical Journal Letters'' (''ApJL''), established in 1967 by Subrahmanyan Chandrasekhar as Part 2 of ''The Astrophysical Journal'', is now a separate journal focusing on the rapid publication of high-impact astronomical research. The three journals were published by the University of Chicago Press for the American Astronomical Society until, in January 2009, publication was transferred to IOP Publis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
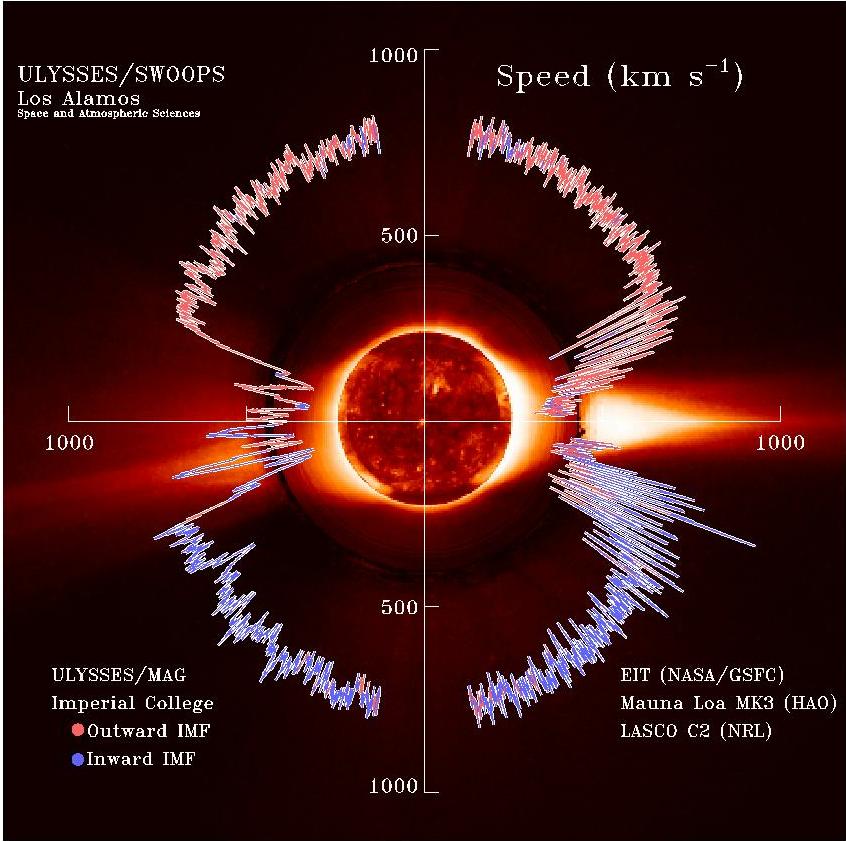
Solar Wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the solar wind plasma also includes a mixture of particle species found in the solar plasma: trace amounts of heavy ions and atomic nuclei of Chemical element, elements such as carbon, nitrogen, oxygen, neon, magnesium, silicon, sulfur, and iron. There are also rarer traces of some other nuclei and isotopes such as phosphorus, titanium, chromium, and nickel's isotopes 58Ni, 60Ni, and 62Ni. Superimposed with the solar-wind plasma is the interplanetary magnetic field. The solar wind varies in density, temperature and speed over time and over Solar coordinate systems#Heliographic, solar latitude and longitude. Its particles can escape the Sun's gravity because of their high energy resulting from the high temperature of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
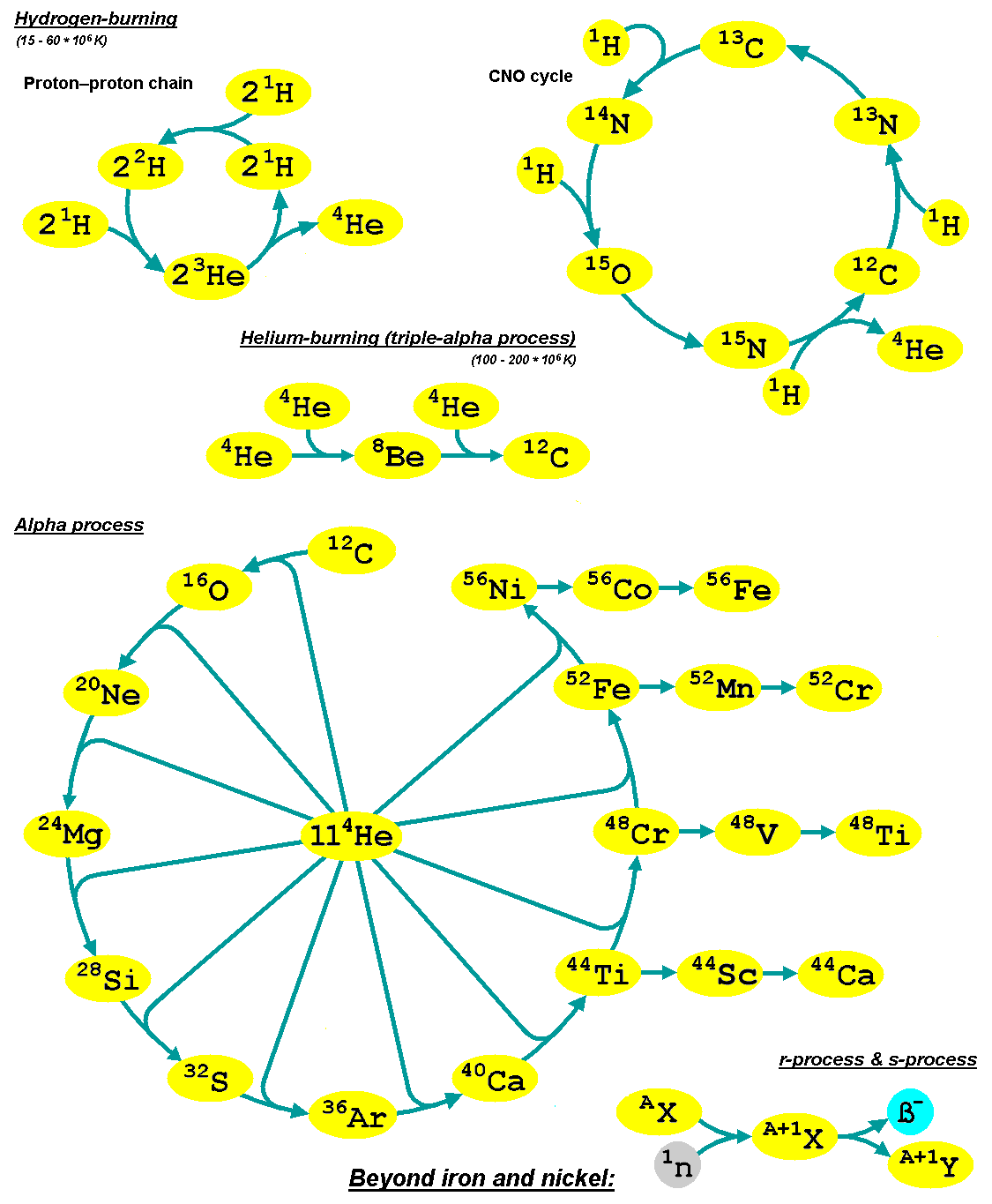
Alpha Process
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae. Both processes are preceded by hydrogen fusion, which produces the helium that fuels both the triple-alpha process and the alpha ladder processes. After the triple-alpha process has produced enough carbon, the alpha-ladder begins and fusion reactions of increasingly heavy elements take place, in the order listed below. Each step only consumes the product of the previous reaction and helium. The later-stage reactions which are able to begin in any particular star, do so while the prior stage reactions are still under way in outer layers of the star. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stellar Nucleosynthesis
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a predictive theory, it yields accurate estimates of the observed abundances of the elements. It explains why the observed abundances of elements change over time and why some elements and their isotopes are much more abundant than others. The theory was initially proposed by Fred Hoyle in 1946, who later refined it in 1954. Further advances were made, especially to nucleosynthesis by neutron capture of the elements heavier than iron, by Margaret and Geoffrey Burbidge, William Alfred Fowler and Fred Hoyle in their famous 1957 B2FH paper, which became one of the most heavily cited papers in astrophysics history. Stars evolve because of changes in their composition (the abundance of their constituent elements) over their lifespans, f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium-40
Potassium-40 (K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 parts-per notation, ppm) of natural potassium. Potassium-40 undergoes four different types of radioactive decay, including all three main types of beta decay: * Electron emission (β) to calcium-40, Ca with a decay energy of 1.31 electronvolt, MeV at 89.6% probability * Positron emission (β) to argon-40, Ar at 0.001% probability * Electron capture (EC) to Ar followed by a gamma decay emitting a photonAlso called a gamma ray, because it is produced by a transition in the nucleus with an energy of 1.46 MeV at 10.3% probability * Direct electron capture (EC) to the ground state of Ar at 0.1%. Both forms of the electron capture decay release further photons,Also called x-ray, as they are emitted from transitions of electrons when electrons from the outer shells fall into the inner shells to replace the electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar System" and "solar system" structures in theinaming guidelines document. The name is commonly rendered in lower case ('solar system'), as, for example, in the ''Oxford English Dictionary'' an''Merriam-Webster's 11th Collegiate Dictionary''. is the gravitationally bound Planetary system, system of the Sun and the objects that orbit it. It Formation and evolution of the Solar System, formed about 4.6 billion years ago when a dense region of a molecular cloud collapsed, forming the Sun and a protoplanetary disc. The Sun is a typical star that maintains a hydrostatic equilibrium, balanced equilibrium by the thermonuclear fusion, fusion of hydrogen into helium at its stellar core, core, releasing this energy from its outer photosphere. As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactivity (chemistry), reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. It exhibits a metallic luster (mineralogy), luster. It corrosion, corrodes quickly in air to a dull silvery gray, then black tarnish. It does not occur freely in nature, but occurs mainly as pegmatite, pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolysis, electrolytically from a mixture of lithium chloride and potassium chloride. The Atomic nucleus, nucleus of the lithiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the ''atomic'' (also known as ''isotopic'') mass of the atom expressed in daltons. Since protons and neutrons are both baryons, the mass number ''A'' is identical with the baryon number ''B'' of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number ''Z'' gives the number of neutrons (''N'') in the nucleus: . The mass number is written either after the element name or as a superscript to the left of an element's symbol. For example, the most common isotope of carbon is carbon-12, or , which has 6 protons and 6 neutrons. The full isotope symbol would also have the atomic number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it is called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the Atomic nucleus, nucleus of an element with an atomic number lower than 95. All known (see: Island of stability) synthetic elements are unstable, but they radioactive decay, decay at widely varying rates; the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were first created artificially are strictly speaking not ''synthetic'' because they were later found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |