|
Stalagmites
A stalagmite (, ; ; ) is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist of lava, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). The corresponding formation hanging down from the ceiling of a cave is a stalactite. Formation and type Limestone stalagmites The most common stalagmites are speleothems, which usually form in limestone caves. Stalagmite formation occurs only under certain pH conditions within the cavern. They form through deposition of calcium carbonate and other minerals, which is precipitated from mineralized water solutions. Limestone is the chief form of calcium carbonate rock, which is dissolved by water that contains carbon dioxide, forming a calcium bicarbonate solution in caverns. The partial pressure of carbon dioxide in the water must be greater than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Speleothem
A speleothem (; ) is a geological formation made by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatic proxies. Chemical and physical characteristics More than 300 variations of cave mineral deposits have been identified. The vast majority of speleothems are calcareous, composed of calcium carbonate (CaCO3) minerals (calcite or aragonite). Less commonly, speleothems are made of calcium sulfate ( gypsum or mirabilite) or opal. Speleothems of pure calcium carbonate or calcium sulfate are translucent and colorless. The presence of iron oxide or copper provides a reddish brown color. The presence of manganese oxide can create darker colors such as black or dark brown. Speleothems can also b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalactite
A stalactite (, ; , ) is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension (chemistry), suspension, or is capable of being melting, melted, may form a stalactite. Stalactites may be composed of lava, minerals, mud, peat, pitch (resin), pitch, sand, Geyserite, sinter, and amberat (crystallized urine of pack rats). A stalactite is not necessarily a speleothem, though speleothems are the most common form of stalactite because of the abundance of limestone caves. The corresponding formation on the floor of the cave is known as a stalagmite. Formation and type Limestone stalactites The most common stalactites are speleothems, which occur in limestone caves. They form through Deposition (geology), deposition of calcium carbonate and other minerals, which is precipitated from mineralized water Solution (chemistry), solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lava Tube
A lava tube, more rarely called a pyroduct, is a 'roofed conduit through which molten lava travels away from its vent'. If lava in the tube drains out, it will leave an empty cave. Lava tubes are common in low-viscosity volcanic systems. Lava tubes are important as they are able to transport molten lava much further away from the eruptive vent than lava channels. A tube-forming lava flow can emplace on longer distance due to the presence of a solid crust protecting the molten lava from atmospheric cooling. Lava tubes are often considered when preparing hazard maps or managing an eruptive crisis. Formation A lava tube is a type of lava cave formed when a low-viscosity lava flow develops a continuous and hard crust, which thickens and forms a roof above the still-flowing lava stream. Three main formation mechanisms have been described: (1) roofing over a lava channel, (2) pāhoehoe lobe extension or (3) lava flow inflation. # When it erupts from a vent, lava usually flow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are '' hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Obsidian
Obsidian ( ) is a naturally occurring volcanic glass formed when lava extrusive rock, extruded from a volcano cools rapidly with minimal crystal growth. It is an igneous rock. Produced from felsic lava, obsidian is rich in the lighter elements such as silicon, oxygen, aluminium, sodium, and potassium. It is commonly found within the margins of rhyolite, rhyolitic lava flows known as obsidian flows. These flows have a high content of silicon dioxide, silica, giving them a high viscosity. The high viscosity inhibits the atomic diffusion, diffusion of atoms through the lava, which inhibits the first step (nucleation) in the formation of mineral crystals. Together with rapid cooling, this results in a natural glass forming from the lava. Obsidian is hard, Brittleness, brittle, and amorphous; it therefore Fracture (mineralogy)#Conchoidal fracture, fractures with sharp edges. In the past, it was used to manufacture cutting and piercing tools, and it has been used experimentally as s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
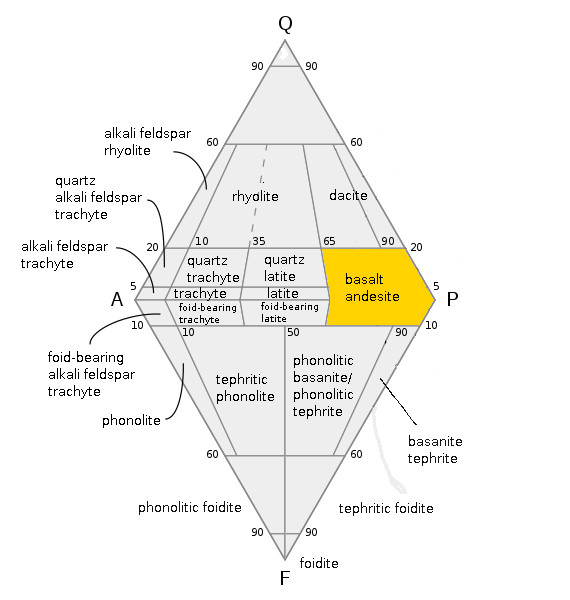
Basalt
Basalt (; ) is an aphanite, aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron (mafic lava) exposed at or very near the planetary surface, surface of a terrestrial planet, rocky planet or natural satellite, moon. More than 90% of all volcanic rock on Earth is basalt. Rapid-cooling, fine-grained basalt is chemically equivalent to slow-cooling, coarse-grained gabbro. The eruption of basalt lava is observed by geologists at about 20 volcanoes per year. Basalt is also an important rock type on other planetary bodies in the Solar System. For example, the bulk of the plains of volcanism on Venus, Venus, which cover ~80% of the surface, are basaltic; the lunar mare, lunar maria are plains of flood-basaltic lava flows; and basalt is a common rock on the surface of Mars. Molten basalt lava has a low viscosity due to its relatively low silica content (between 45% and 52%), resulting in rapidly moving lava flo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Gerridae, water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to Cohesion (chemistry), cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
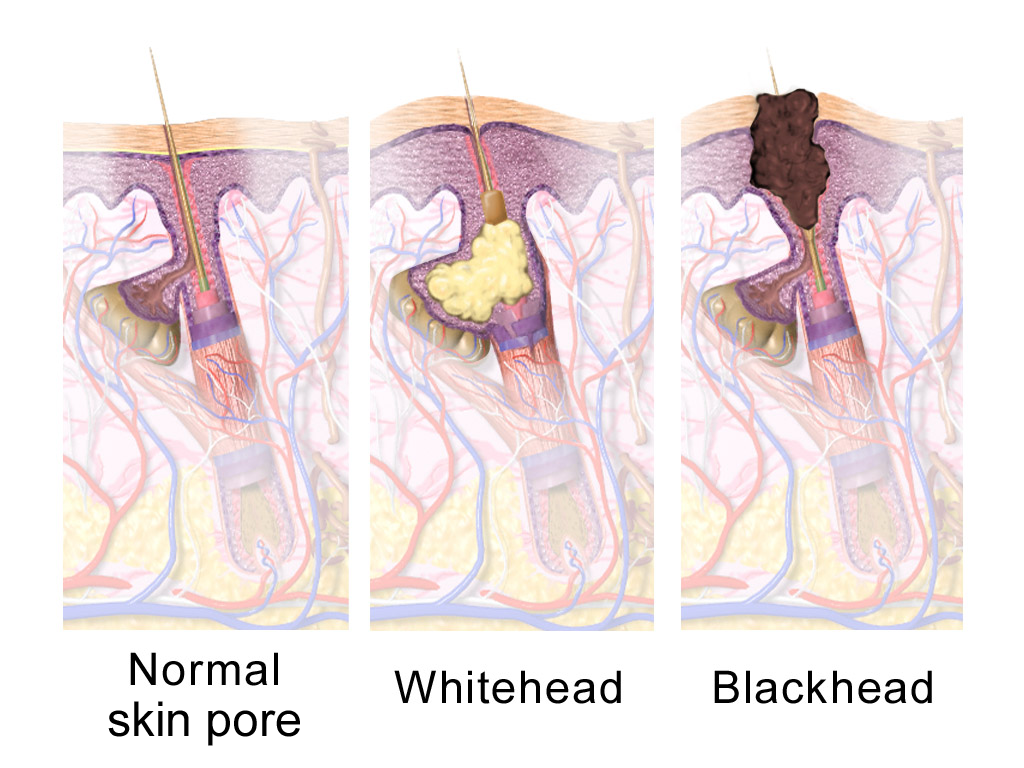
Sebum
A sebaceous gland or oil gland is a microscopic exocrine gland in the skin that opens into a hair follicle to secrete an oily or waxy matter, called sebum, which lubricates the hair and skin of mammals. In humans, sebaceous glands occur in the greatest number on the face and scalp, but also on all parts of the skin except the palms of the hands and soles of the feet. In the eyelids, meibomian glands, also called tarsal glands, are a type of sebaceous gland that secrete a special type of sebum into tears. Surrounding the female nipples, areolar glands are specialized sebaceous glands for lubricating the nipples. Fordyce spots are benign, visible, sebaceous glands found usually on the lips, gums and inner cheeks, and genitals. Structure Location In humans, sebaceous glands are found throughout all areas of the skin, except the palms of the hands and soles of the feet. There are two types of sebaceous glands: those connected to hair follicles and those that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). In respiratory physiology, the partial pressure of a dissolved gas in liquid (such as oxygen in arterial blood) is also defined as the partial pressure of that gas as it would be undissolved in gas phase yet in equilibrium with the liquid. This concept is also known as blood gas tension. In this sense, the diffusion of a gas liquid is said to be driven by differences in partial pressure (not concentration). In chemistry and thermodynamics, this concept is generalized to non-ideal gases and instead called fugacity. The partial pressure of a gas is a measure of its Thermodynamic activity, thermodynamic activity. Gases dissolve, diffuse, and react accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Bicarbonate
Calcium bicarbonate, also called calcium hydrogencarbonate, has the chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (), and carbonate () ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water. All waters in contact with the atmosphere absorb carbon dioxide, and as these waters come into contact with rocks and sediments they acquire metal ions, most commonly calcium and magnesium, so most natural waters that come from streams, lakes, and especially wells, can be regarded as dilute solutions of these bicarbonates. These hard waters tend to form carbonate scale in pipes and boilers, and they react with soaps to form an undesirable scum. Attempts to prepare compounds such as solid calcium bicarbonate by evaporating its solution t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |