|
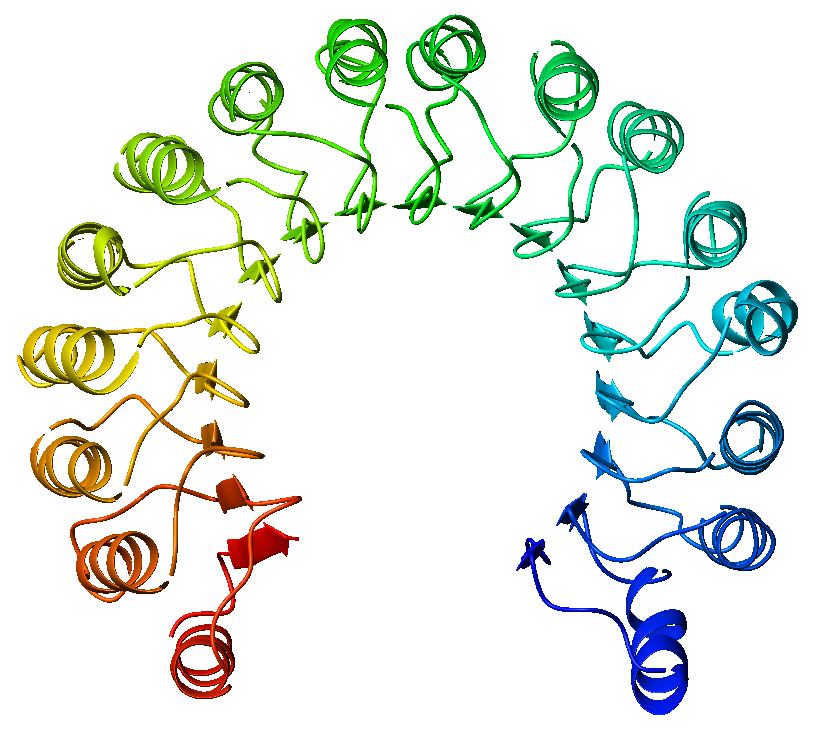
Slit (protein)
Slit is a family of secreted extracellular matrix proteins which play an important signalling role in the neural development of most bilaterians (animals with bilateral symmetry). While lower animal species, including insects and nematode worms, possess a single Slit gene, humans, mice and other vertebrates possess three Slit homologs: Slit1, Slit2 and Slit3. Human ''Slits'' have been shown to be involved in certain pathological conditions, such as cancer and inflammation. The ventral midline of the central nervous system is a key place where axons can either decide to cross and laterally project or stay on the same side of the brain. The main function of Slit proteins is to act as midline repellents, preventing the crossing of longitudinal axons through the midline of the central nervous system of most bilaterian animal species, including mice, chickens, humans, insects, nematode worms and planarians. It also prevents the recrossing of commissural axons. Its canonical re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Commissural Axon
A commissure () is the location at which two objects abut or are joined. The term is used especially in the fields of anatomy and biology. * The most common usage of the term refers to the brain's commissures, of which there are at least nine. Such a commissure is a bundle of commissural fibers as a tract that crosses the midline at its level of origin or entry (as opposed to a decussation of fibers that cross obliquely). The nine are the anterior commissure, posterior commissure, corpus callosum, commissure of fornix (hippocampal commissure), habenular commissure, ventral supraoptic decussation, Meynert's commissure, anterior hypothalamic commissure of Gasner, and the interthalamic adhesion. They consist of fibre tracts that connect the two cerebral hemispheres and span the longitudinal fissure. In the spinal cord, there are the anterior white commissure, and the gray commissure. ''Commissural neurons'' refer to neuronal cells that grow their axons across the midline of the ner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks Covalent bond, bonds. Proteases are involved in numerous biological pathways, including Digestion#Protein digestion, digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently convergent evolution, evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Classification Based on catalytic residue Proteases can be classified into seven broad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine Knot
A cystine knot is a protein structural motif containing three disulfide bridges (formed from pairs of cysteine residues). The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane-like substructure. The cystine knot motif stabilizes protein structure and is conserved in proteins across various species. There are three types of cystine knot, which differ in the topology of the disulfide bonds: * Growth factor cystine knot (GFCK) * Inhibitor cystine knot (ICK), common in spider and snail toxins * Cyclic cystine knot, or cyclotide The growth factor cystine knot was first observed in the structure of nerve growth factor (NGF), solved by X-ray crystallography and published in 1991.; The GFCK is present in four superfamilies. These include nerve growth factor, transforming growth factor beta (TGF-β), platelet-derived growth factor, and glycoprotein hormones including human chorionic gonadotropin. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
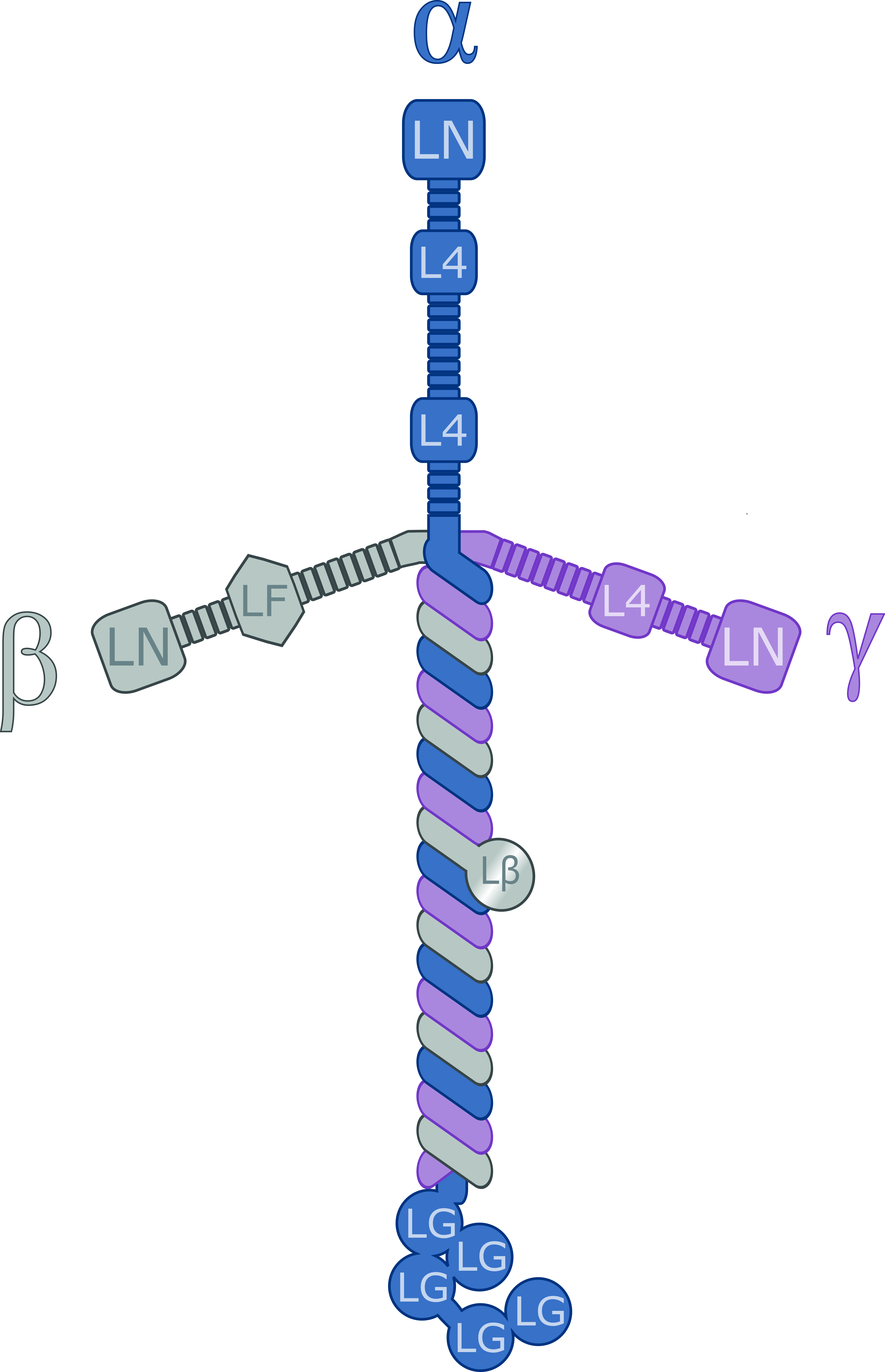
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major constituents of the basement membrane, namely the basal lamina (the protein network foundation for most cells and organs). Laminins are vital to biological activity, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa) and possess three different chains (α, β, and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition, e.g. laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect, composing a cruciform structure that is able to bind to other molecules of the extracellular matrix and cell membrane. The three short arms have an affinity for binding to other laminin molecules, conducing sheet formation. The long ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine-rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe tertiary structure, fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These Protein tandem repeats, tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta sheet, beta strand-turn (biochemistry), turn-alpha helix structure, and the assembled structural domain, domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Growth Cones
Growth may refer to: Biology *Auxology, the study of all aspects of human physical growth *Bacterial growth *Cell growth *Growth hormone, a peptide hormone that stimulates growth *Human development (biology) *Plant growth *Secondary growth, growth that thickens woody plants *A tumor or other such neoplasm Economics * Economic growth, the increase in the inflation-adjusted market value of the goods and services * Growth investing, a style of investment strategy focused on capital appreciation Mathematics * Exponential growth, also called geometric growth * Hyperbolic growth * Linear function, Linear growth, refers to two distinct but related notions * Logistic function, Logistic growth, characterized as an S curve Social science * Developmental psychology * Erikson's stages of psychosocial development * Human development (humanity) * Personal development * Population growth Other uses * Growth (film), ''Growth'' (film), a 2010 American horror film * Izaugsme (''Growth''), a Latv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster
''Drosophila melanogaster'' is a species of fly (an insect of the Order (biology), order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly", or "banana fly". In the wild, ''D. melanogaster'' are attracted to rotting fruit and fermenting beverages, and are often found in orchards, kitchens and pubs. Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, ''D. melanogaster'' continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and Life history theory, life history evolution. ''D. melanogaster'' was the first animal to be Fruit flies in space, launched into space in 1947. As of 2017, six Nobel Prizes have been awarded to drosophilists for their work using the insect. ''Drosophila melanogaster'' is typically used in research owing to its rapid life cycle, relatively simple genetics with on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pioneer Axon
Pioneer axon is the classification given to axons that are the first to grow in a particular region. They originate from pioneer neurons, and have the main function of laying down the initial growing path that subsequent growing axons, dubbed follower axons, from other neurons will eventually follow. Several theories relating to the structure and function of pioneer axons are currently being explored. The first theory is that pioneer axons are specialized structures, and that they play a crucial role in guiding follower axons. The second is that pioneer axons are no different from follower axons, and that they play no role in guiding follower axons. Anatomically, there are no differences between pioneer and follower axons, although there are morphological differences. The mechanisms of pioneer axons and their role in axon guidance is currently being explored. In addition, many studies are being conducted in model organisms, such grasshoppers, zebrafish, and fruit flies to study ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |