|
Simple Modular Architecture Research Tool
Simple Modular Architecture Research Tool (SMART) is a biological database that is used in the identification and analysis of protein domains within protein sequences. SMART uses hidden Markov model, profile-hidden Markov models built from multiple sequence alignments to detect protein domains in protein sequences. The most recent release of SMART contains 1,204 domain models. Data from SMART was used in creating the Conserved Domain Database collection and is also distributed as part of the InterPro database. The database is hosted by the European Molecular Biology Laboratory in Heidelberg. References External links SMART web site Protein structure Protein classification Protein databases {{bioinformatics-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SMART Logo
''SMart'' was a British CBBC television programme based on art, which began in 1994 and ended in 2009. The programme was recorded at BBC Television Centre in London. Previously it had been recorded in Studio A at Pebble Mill Studios in Birmingham. The format is similar to the Tony Hart programmes ''Take Hart'' and ''Hartbeat''. The show was revamped into an hour-long show in 2007; from 1994 to 2006 it was previously a 25-minute show. From 1994 to 2005, the show also featured Morph (character), Morph, originally from ''Take Hart''. The series run featured 199 episodes, last airing on 11 August 2011. Production The BBC noticed the success of ''Art Attack'' with Neil Buchanan for CITV which started in 1990 and decided to create their own art show that was accessible to children similar to ''Art Attack''. The original theme tune was composed by Kjartan Poskitt, famous for the ''Murderous Maths'' series of books. From 2003, a different tune was used, written by Steve Brown (composer) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Identification Scheme
In metadata, an identification scheme is used to identify unique records in a set. If a data element is used to identify a record within a data set, the data element uses the Identifier representation term. An identification scheme should be contrasted with a classification scheme. Classification schemes are used to classify individual records into categories. Many records in a data set may be in a single category. See also * Classification scheme * Metadata * Representation term A representation term is a word, or a combination of words, that semantically represent the data type (value domain) of a data element. A representation term is commonly referred to as a ''class word'' by those familiar with data dictionaries. ISO ... Metadata References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
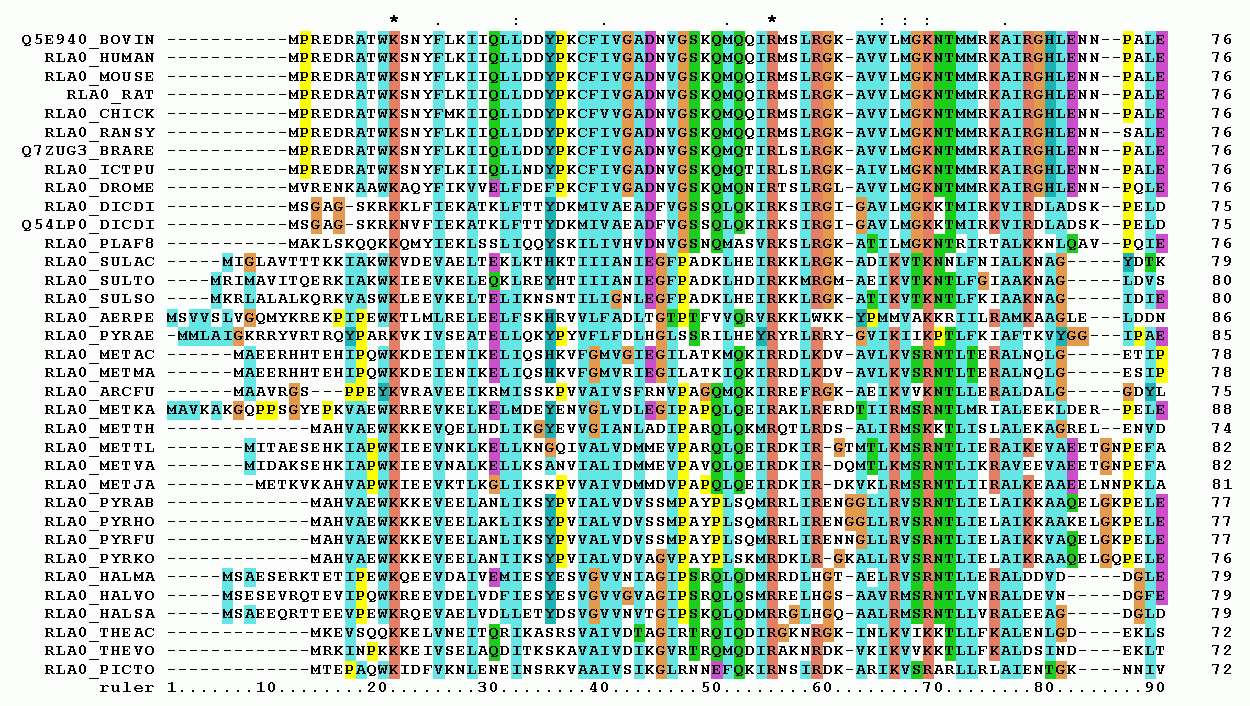
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Molecular Biology Laboratory
The European Molecular Biology Laboratory (EMBL) is an intergovernmental organization dedicated to molecular biology research and is supported by 29 member states, two prospect member states, and one associate member state. EMBL was created in 1974 and is funded by public research money from its member states. Research at EMBL is conducted by more than 110 independent research groups and service teams covering the spectrum of molecular biology. The Laboratory operates from six sites: the main laboratory in Heidelberg (Germany), and sites in Barcelona (Spain), Grenoble (France), Hamburg (Germany), Hinxton (the European Bioinformatics Institute (EBI), in England), and Rome (Italy). EMBL groups and laboratories perform basic research in molecular biology and molecular medicine as well as train scientists, students, and visitors. The organization aids in the development of services, new instruments and methods, and technology in its member states. Israel is the only full member stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Database
Biological databases are libraries of biological sciences, collected from scientific experiments, published literature, high-throughput experiment technology, and computational analysis. They contain information from research areas including genomics, proteomics, metabolomics, microarray gene expression, and phylogenetics. Information contained in biological databases includes gene function, structure, localization (both cellular and chromosomal), clinical effects of mutations as well as similarities of biological sequences and structures. Biological databases can be classified by the kind of data they collect (see below). Broadly, there are molecular databases (for sequences, molecules, etc.), functional databases (for physiology, enzyme activities, phenotypes, ecology etc), taxonomic databases (for species and other taxonomic ranks), images and other media, or specimens (for museum collections etc.) Databases are important tools in assisting scientists to analyze and explain a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hidden Markov Model
A hidden Markov model (HMM) is a Markov model in which the observations are dependent on a latent (or ''hidden'') Markov process (referred to as X). An HMM requires that there be an observable process Y whose outcomes depend on the outcomes of X in a known way. Since X cannot be observed directly, the goal is to learn about state of X by observing Y. By definition of being a Markov model, an HMM has an additional requirement that the outcome of Y at time t = t_0 must be "influenced" exclusively by the outcome of X at t = t_0 and that the outcomes of X and Y at t < t_0 must be conditionally independent of at given at time . Estimation of the parameters in an HMM can be performed using maximum likelihood estimation. For linear chain HMMs, the Baum–Welch algorithm can be used to estimate parameters. Hidden Markov models are known for their applications to thermodynamics, statistical mechanics, physics, chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multiple Sequence Alignment
Multiple sequence alignment (MSA) is the process or the result of sequence alignment of three or more biological sequences, generally protein, DNA, or RNA. These alignments are used to infer evolutionary relationships via phylogenetic analysis and can highlight homologous features between sequences. Alignments highlight mutation events such as point mutations (single amino acid or nucleotide changes), insertion mutations and deletion mutations, and alignments are used to assess sequence conservation and infer the presence and activity of protein domains, tertiary structures, secondary structures, and individual amino acids or nucleotides. Multiple sequence alignments require more sophisticated methodologies than pairwise alignments, as they are more computationally complex. Most multiple sequence alignment programs use heuristic methods rather than global optimization because identifying the optimal alignment between more than a few sequences of moderate length is prohibiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
InterPro
InterPro is a database of protein families, protein domains and functional sites in which identifiable features found in known proteins can be applied to new protein sequences in order to functionally characterise them. The contents of InterPro consist of diagnostic signatures and the proteins that they significantly match. The signatures consist of models (simple types, such as regular expressions or more complex ones, such as Hidden Markov models) which describe protein families, domains or sites. Unknown sequences are searched to create homology models. Each of the member databases of InterPro contributes towards a different niche, from very high-level, structure-based classifications ( SUPERFAMILY and CATH-Gene3D) through to quite specific sub-family classifications ( PRINTS and PANTHER). InterPro's intention is to provide a one-stop-shop for protein classification, where all the signatures produced by the different member databases are placed into entries within the Inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid monomer may also be called a ''residue'', which indicates a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions, such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |