|
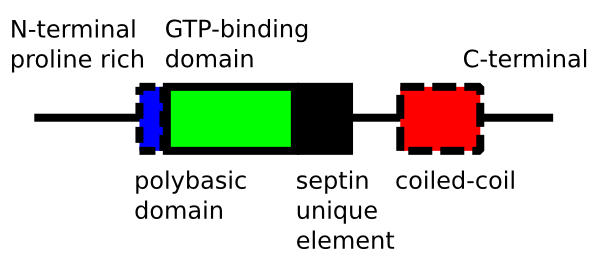
Septin
Septins are a group of guanosine triphosphate, GTP-molecular binding, binding proteins gene expression, expressed in all eukaryote, eukaryotic cells except plants. Different septins form protein complexes with each other. These complexes can further assemble into filaments, rings and gauzes. Assembled as such, septins function in cells by localizing other proteins, either by providing a scaffold to which proteins can attach, or by forming a barrier preventing the diffusion of molecules from one compartment of the cell to another, or in the cell cortex as a barrier to the diffusion of membrane-bound proteins. Septins have been implicated in the localization of cellular processes at the site of cell division, and at the cell membrane at sites where specialized structures like cilium, cilia or flagella are attached to the cell body. In yeast cells, they compartmentalize parts of the cell and build scaffolding to provide structural support during cell division at the septum (cell bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth and or disassembly depending on the cell's requirements. Cytoskeleton can perform many functions. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), the segregation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Septum (cell Biology)
A septum in cell biology is the new cell wall that forms between two daughter cells as a result of cell division. Cell division is an extremely complex process that contains four different subprocesses. These processes included the growth of a cell, DNA replication, the process of allocating replicated chromosomes to daughter cells, and septum formation. Ultimately, the septum is the crucial ending to mitosis, meiosis, and the division of bacterial cells. The formation of the septum (a new cell wall) allows the two daughter cells to be separate from one another and perform their respective functions independently. Composition In ''Schizosaccharomyces pombe'', the primary septum is composed of linear β(1,3)-D-glucan, β(1,6) branches, and α(1,3)-D-glucan. The secondary septum in Schizosaccharomyces pombe is composed of β(1,6)-D-glucan, β(1,6) branches, and α(1,3)-D-glucan. The synthesis of linear β(1,3)-D-glucan for the primary septum is done by the enzyme β(1,3)-D-glucan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one of the kingdom (biology)#Six kingdoms (1998), traditional eukaryotic kingdoms, along with Animalia, Plantae, and either Protista or Protozoa and Chromista. A characteristic that places fungi in a different kingdom from plants, bacteria, and some protists is chitin in their cell walls. Fungi, like animals, are heterotrophs; they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Fungi do not photosynthesize. Growth is their means of motility, mobility, except for spores (a few of which are flagellated), which may travel through the air or water. Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
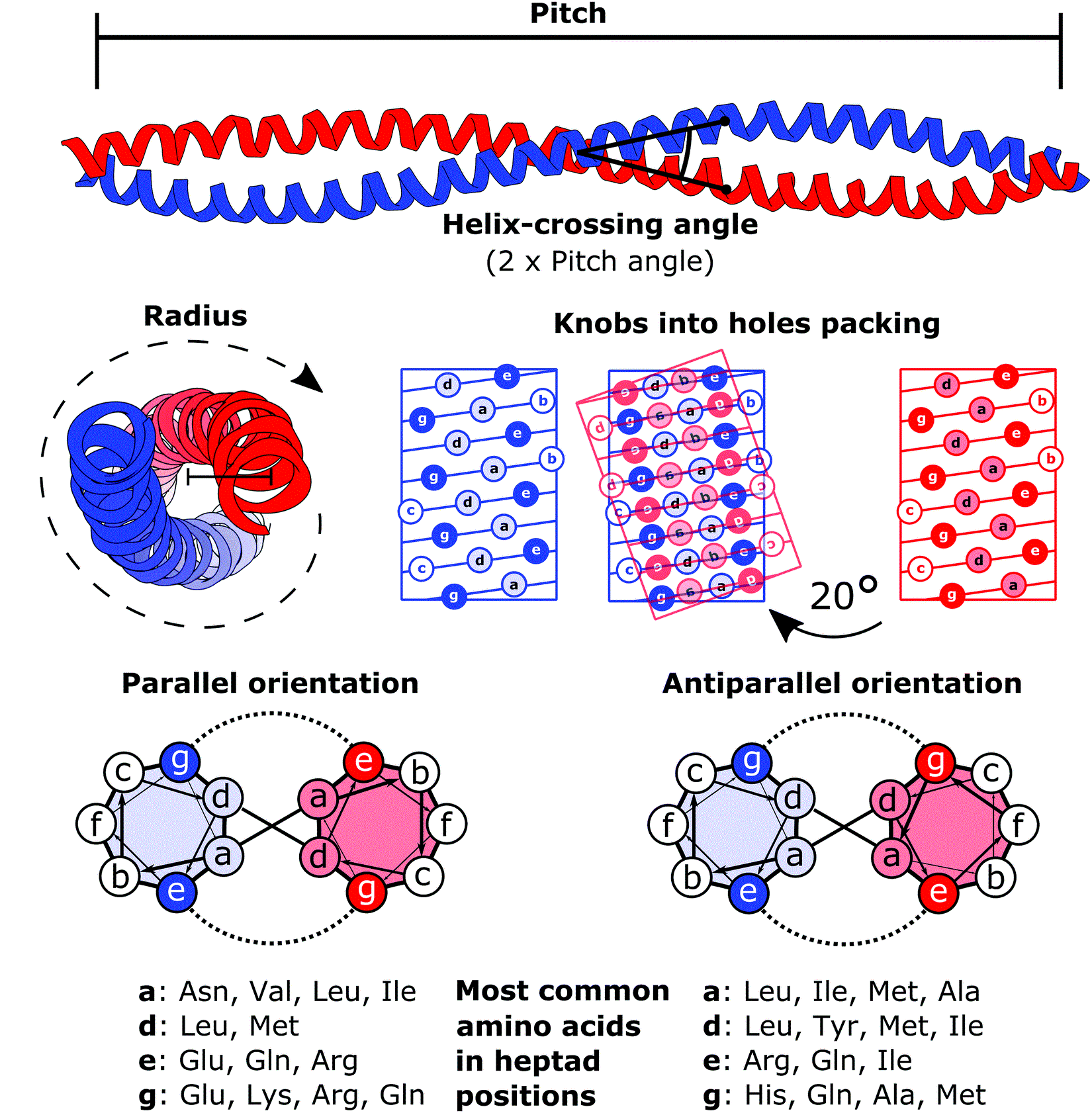
Coiled Coil
A coiled coil is a structural motif in proteins in which two to seven alpha-helices are coiled together like the strands of a rope. ( Dimers and trimers are the most common types.) They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
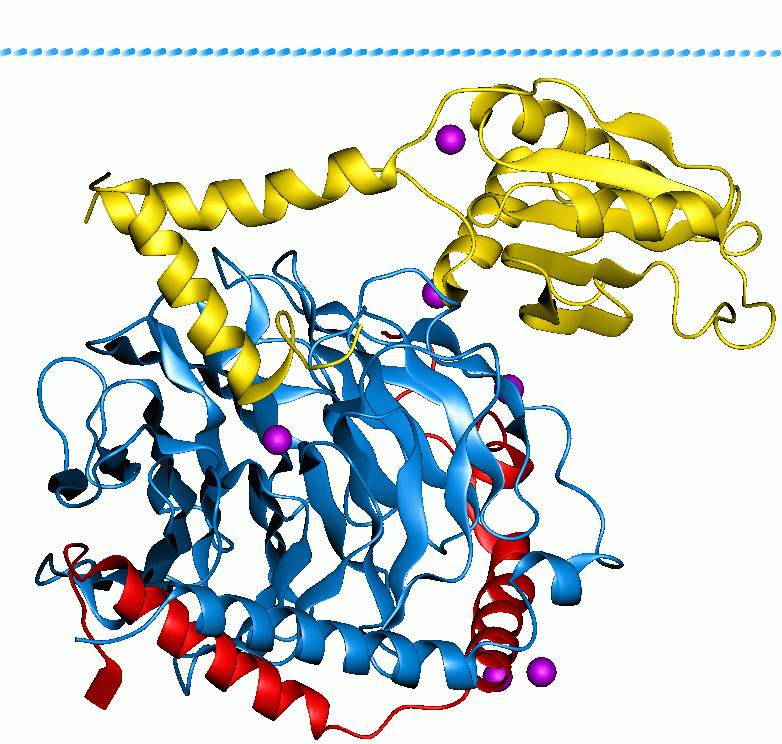
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a Protein family, family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell (biology), cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein protein complex, complexes. The latter class of complexes is made up of ''G alpha subunit, alpha'' (Gα), ''beta'' (Gβ) and ''gamma'' (Gγ) protein subunit, subunits. In addition, the beta and gamma subunits can form a stable Protein dimer, dimeric complex re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''Asn, followed by anything but Pro, followed by either Ser or Thr, followed by anything but Pro residue''. Overview When a sequence motif appears in the exon of a gene, it may encode the " structural motif" of a protein; that is a stereotypical element of the overall structure of the protein. Nevertheless, motifs need not be associated with a distinctive secondary structure. " Noncoding" sequences are not translated into proteins, and nucleic acids with such motifs need not deviate from the typical shape (e.g. the "B-form" DNA double helix). Outside of gene exons, there exist regulatory sequence motifs and motifs within the " junk", such as satellite DNA. Some of these are believed to affect the shape of nucleic acids (see for example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding (molecular)
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding. In some cases, the associations can be quite strong—for example, the protein streptavidin and the vitamin biotin have a dissociation constant (reflecting the ratio between bound and free biotin) on the order of 10−14—and so the reactions are effectively irreversible. The result of molecular binding is sometimes the formation of a molecular complex in which the attractive forces holding the components together are generally non-covalent, and thus are normally energetically weaker than covalent bonds. Molecular binding occurs in biological complexes (e.g., between pairs or sets of proteins, or between a protein and a small molecule ligand it binds) and also in abiologic chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoinositide
Phosphatidylinositol or inositol phospholipid is a biomolecule. It was initially called "inosite" when it was discovered by Léon Maquenne and Johann Joseph von Scherer in the late 19th century. It was discovered in bacteria but later also found in eukaryotes, and was found to be a signaling molecule. The biomolecule can exist in 9 different isomers. It is a lipid which contains a phosphate group, two fatty acid chains, and one inositol sugar molecule. Typically, the phosphate group has a negative charge (at physiological pH values). As a result, the molecule is amphiphilic. The production of the molecule is limited to the endoplasmic reticulum. History of phospatidylinositol Phosphatidylinositol (PI) and its derivatives have a rich history dating back to their discovery by Johann Joseph von Scherer and Léon Maquenne in the late 19th century. Initially known as "inosite" based on its sweet taste, the isolation and characterization of inositol laid the groundwork for unders ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |