|
Selenium In Biology
Selenium is an essential mineral micronutrient for animals, though it is toxic in large doses. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−). Functions in animals Selenium is an essential micronutrient in mammals, but is also recognized as toxic in excess. Selenium exerts its biological functions through selenoproteins, which contain the amino acid selenocysteine. Twenty-five selenoproteins are encoded in the human genome. Glutathione peroxidase The glutathione peroxidase family of enzymes (abbreviated GSH-Px) catalyze reduction of hydrogen peroxide and organic hydroperoxides: :2GSH + H2O2 → GSSG + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenocysteine Skeletal 3D
Selenocysteine (symbol Sec or U, in older publications also as Se-Cys) is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the sulfur. Selenocysteine is present in several enzymes (for example glutathione peroxidases, tetraiodothyronine 5′ deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases, selenophosphate synthetase 2, methionine-''R''-sulfoxide reductase B1 ( SEPX1), and some hydrogenases). It occurs in all three domains of life, including important enzymes (listed above) present in humans. Selenocysteine was discovered in 1974 by biochemist Thressa Stadtman at the National Institutes of Health. Chemistry Selenocysteine is the Se-analogue of cysteine. It is rarely encountered outside of living tissue (nor is it available commercially) because of its high susceptiblility to air-oxidation. More common is the oxidized derivative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anion () and its Salt (chemistry), salts, and the neutral hydroperoxyl, hydroperoxyl radical (•OOH) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with saturated bond, saturated chemical bonds. Properties The bond length in peroxides is about 1.45 Ångström, Å, and the angles (R = Hydrogen, H, Carbon, C) are about 110° (water-like). Characteristically, the dihedral angles are about 120°. The bond is relatively weak, with a bond dissociation energy of , less than half the strength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the developing embryo. Although not entirely understood, thioredoxin is linked to medicine through their response to reactive oxygen species (ROS). In plants, thioredoxins regulate a spectrum of critical functions, ranging from photosynthesis to growth, flowering and the development and germination of seeds. Thioredoxins play a role in Cell signaling, cell-to-cell communication. Occurrence They are found in nearly all known organisms and are essential for life in mammals. Function The primary function of thioredoxin (Trx) is the reduction of oxidized cysteine residues and the cleavage of disulfide bonds. Multiple in vitro subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups. In inorganic chemistry, the anion appears in a few rare minerals, but the functional group has tremendous importance in biochemistry. Disulfide bridges formed between thiol groups in two cysteine residues are an important component of the tertiary and quaternary structure of proteins. Compounds of the form are usually called ''persulfides'' instead. Organic disulfides Structure Disulfides have a C–S–S–C dihedral angle approaching 90°. The S–S bond length is 2.03 Å in diphenyl disulfide, similar to that in elemental sulfur. Disulfides are usually symmetric but they can also be unsymmetric. Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organosulfur chemistry are symmetrical disulfides. Unsymme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin Reductase
Thioredoxin reductases (TR, TrxR) () are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Bacterial TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a flavin adenine dinucleotide, FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond. Cellular role Thioredoxin reductases are enzymes that catalyze the reduction of thioredoxin and hence they are a central component in the thioredoxin system. Together with thioredoxin (Trx) and NADPH this system's most general description is as a system for reducing disulfide bonds in cells. Electrons are taken from NADPH via TrxR and are transferred to the active site of Trx, which goes on to reduce protein disulfides or other substrates. The Trx system exists in all living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formate Dehydrogenase
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD+ in formate:NAD+ oxidoreductase () or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (). This family of enzymes has attracted attention as inspiration or guidance on methods for the carbon dioxide fixation, relevant to global warming. Function NAD-dependent formate dehydrogenases are important in methylotrophic yeast and bacteria, being vital in the catabolism of C1 compounds such as methanol. The cytochrome-dependent enzymes are more important in anaerobic metabolism in prokaryotes. For example, in '' E. coli'', the formate:ferricytochrome-b1 oxidoreductase is an intrinsic membrane protein with two subunits and is involved in anaerobic nitrate respiration. NAD-dependent reaction Formate + NAD+ CO2 + NADH + H+ Cytochrome-dependent reaction Formate + 2 ferricytochrome b1 CO2 + 2 ferrocytochrome b1 + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
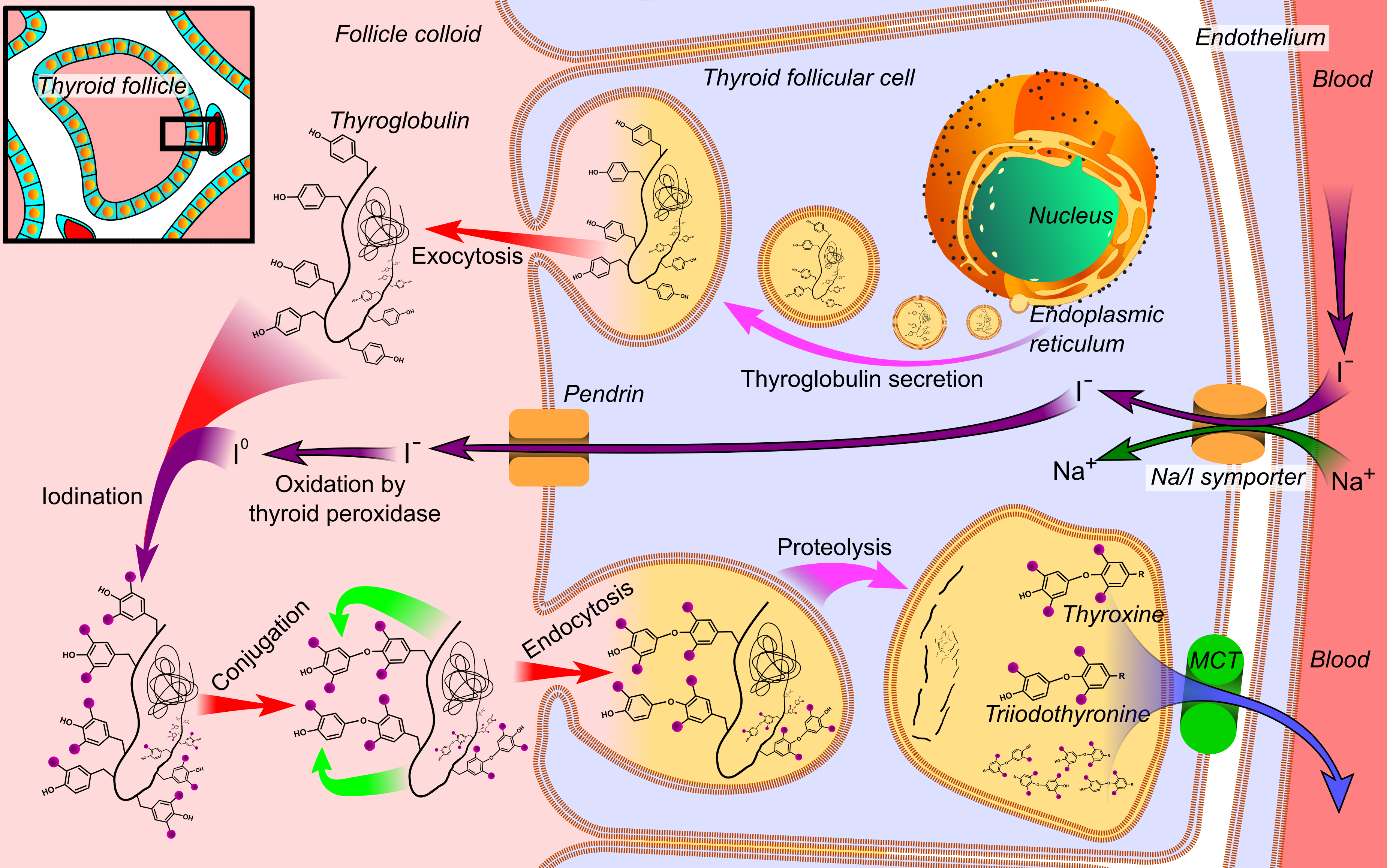
Thyroid Peroxidase
Thyroid peroxidase, also called thyroperoxidase (TPO), thyroid specific peroxidase or iodide peroxidase, is an enzyme expressed mainly in the thyroid where it is secreted into colloid. Thyroid peroxidase oxidizes iodide ions to form iodine atoms for addition onto tyrosine residues on thyroglobulin for the production of thyroxine (T4) or triiodothyronine (T3), the thyroid hormones. In humans, thyroperoxidase is encoded by the ''TPO'' gene. Function Inorganic iodine enters the body primarily as iodide, I−. After entering the thyroid follicle (or thyroid follicular cell) via a Na+/I− symporter (NIS) on the basolateral side, iodide is shuttled across the apical membrane into the colloid via pendrin after which thyroid peroxidase oxidizes iodide to atomic iodine (I) or iodinium (I+). The chemical reactions catalyzed by thyroid peroxidase occur on the outer apical membrane surface and are mediated by hydrogen peroxide. The "organification of iodine", the incorporation of iodine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
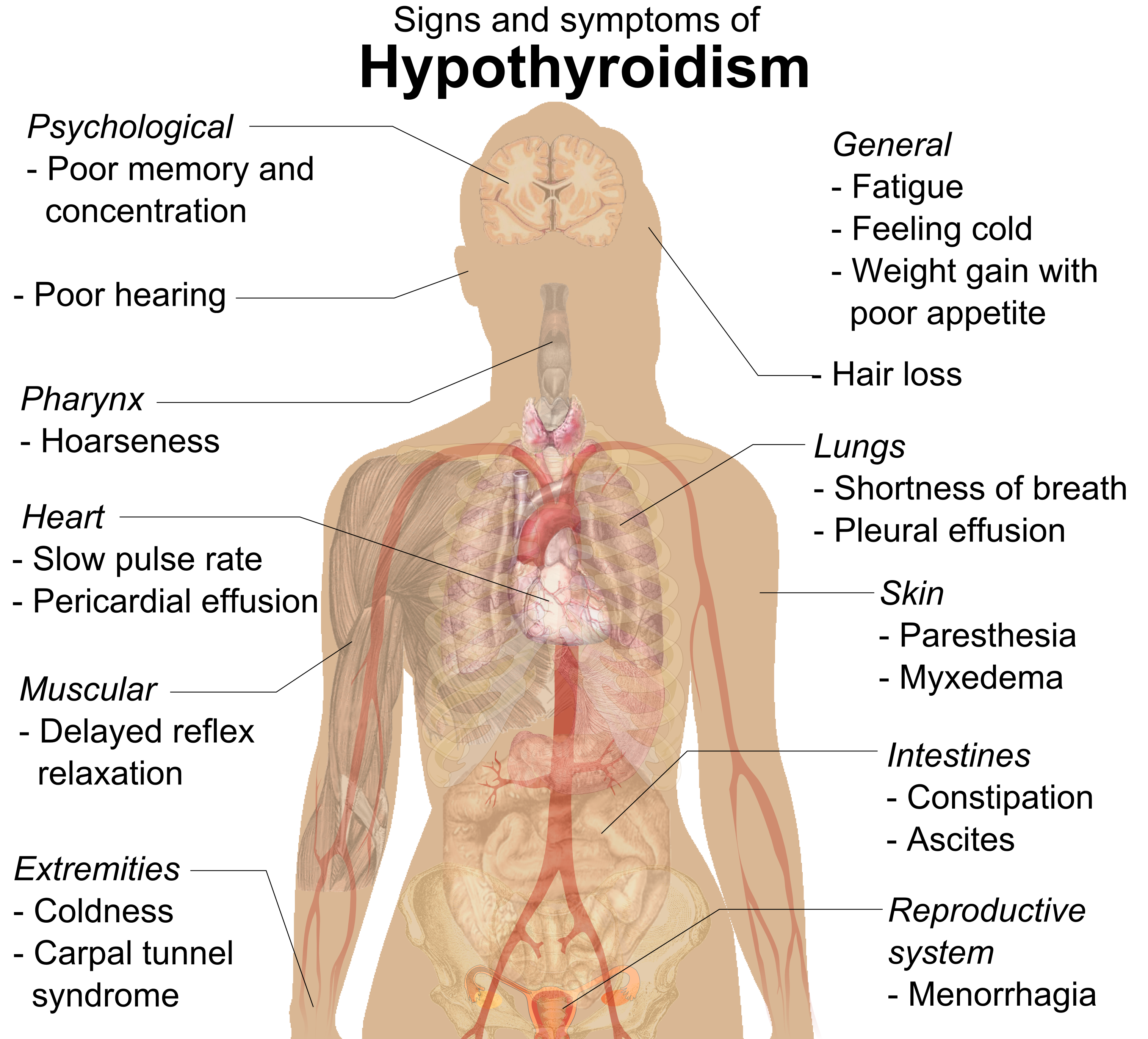
Hashimoto's Thyroiditis
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis, Hashimoto's disease and autoimmune thyroiditis, is an autoimmune disease in which the thyroid gland is gradually destroyed. Early on, symptoms may not be noticed. Over time, the thyroid may enlarge, forming a painless goiter. Most people eventually develop hypothyroidism with accompanying weight gain, fatigue, constipation, hair loss, and general pains. After many years the thyroid typically shrinks in size. Potential complications include thyroid lymphoma. Further complications of hypothyroidism can include high cholesterol, heart disease, heart failure, high blood pressure, myxedema, and potential problems in pregnancy. Hashimoto's thyroiditis is thought to be due to a combination of genetic and environmental factors. Risk factors include a family history of the condition and having another autoimmune disease. Diagnosis is confirmed with blood tests for TSH, thyroxine ( T4), antithyroid autoant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deiodinase
Deiodinase (monodeiodinase) is a peroxidase enzyme that is involved in the activation or deactivation of thyroid hormones. Types Types of deiodinases include: Iodothyronine deiodinases catalyze release of iodine directly from the thyronine hormones. They are selenocysteine-dependent membrane proteins with a catalytic domain resembling peroxiredoxins (Prx). Three related isoforms, deiodinase type I, II, and III, contribute to activation and inactivation of the initially released hormone precursor T4 (thyroxine) into T3 (triiodothyronine) or rT3 ( reverse triiodothyronine) in target cells. The enzymes catalyze a reductive elimination of iodine (the different isoforms attack different thyronine positions), thereby oxidizing themselves similar to Prx, followed by a reductive recycling of the enzyme. Iodotyrosine deiodinase contributes to breakdown of thyroid hormones. It releases iodine, for renewed use, from iodinated tyrosines resulting from catabolism of iodothyronines. Io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thyroid Hormone
File:Thyroid_system.svg, upright=1.5, The thyroid system of the thyroid hormones triiodothyronine, T3 and T4 rect 376 268 820 433 Thyroid-stimulating hormone rect 411 200 849 266 Thyrotropin-releasing hormone rect 297 168 502 200 Hypothalamus rect 66 216 386 256 Anterior pituitary, Anterior pituitary gland rect 66 332 342 374 Negative feedback rect 308 436 510 475 Thyroid, Thyroid gland rect 256 539 563 635 Thyroid hormones rect 357 827 569 856 Catecholamine rect 399 716 591 750 Metabolism desc bottom-left Thyroid hormones are two hormones produced and released by the thyroid gland, triiodothyronine (T3) and thyroxine (T4). They are tyrosine-based hormones that are primarily responsible for regulation of metabolism. T3 and T4 are partially composed of iodine, derived from food. A deficiency of iodine leads to decreased production of T3 and T4, enlarges the thyroid, thyroid tissue and will cause the disease known as simple goitre. The major form of thyroid hormone in the blood ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
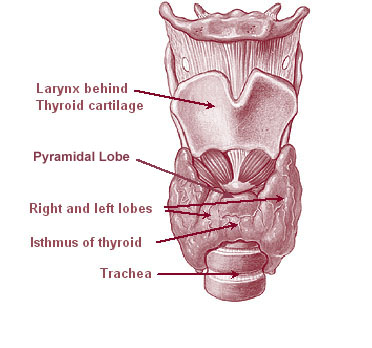
Thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the isthmus (: isthmi). Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones triiodothyronine (T3) and thyroxine (T4)and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis and growth and development in children. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonucleotide Reductase
Ribonucleotide reductase (RNR), also known as ribonucleoside diphosphate reductase, is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. It catalyzes this formation by removing the 2'-hydroxyl group of the ribose ring of nucleoside diphosphates (or triphosphates depending on the class of RNR). This reduction produces deoxyribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of deoxyribonucleic acid, DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms. Furthermore, RNR plays a critical role in regulating the total rate of DNA synthesis so that DNA to cell mass is maintained at a constant ratio during cell division and DNA repair. A somewhat unusual feature of the RNR enzyme is that it catalyzes a reaction that proceeds via a free radical mechanism of action. The substrates for RNR are adenosine diphosphate, ADP, guanosine diphosphate, GDP, cytidine diphosphate, CDP and uridine diphosphate, UDP. dTDP ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |