|
Saltpetre
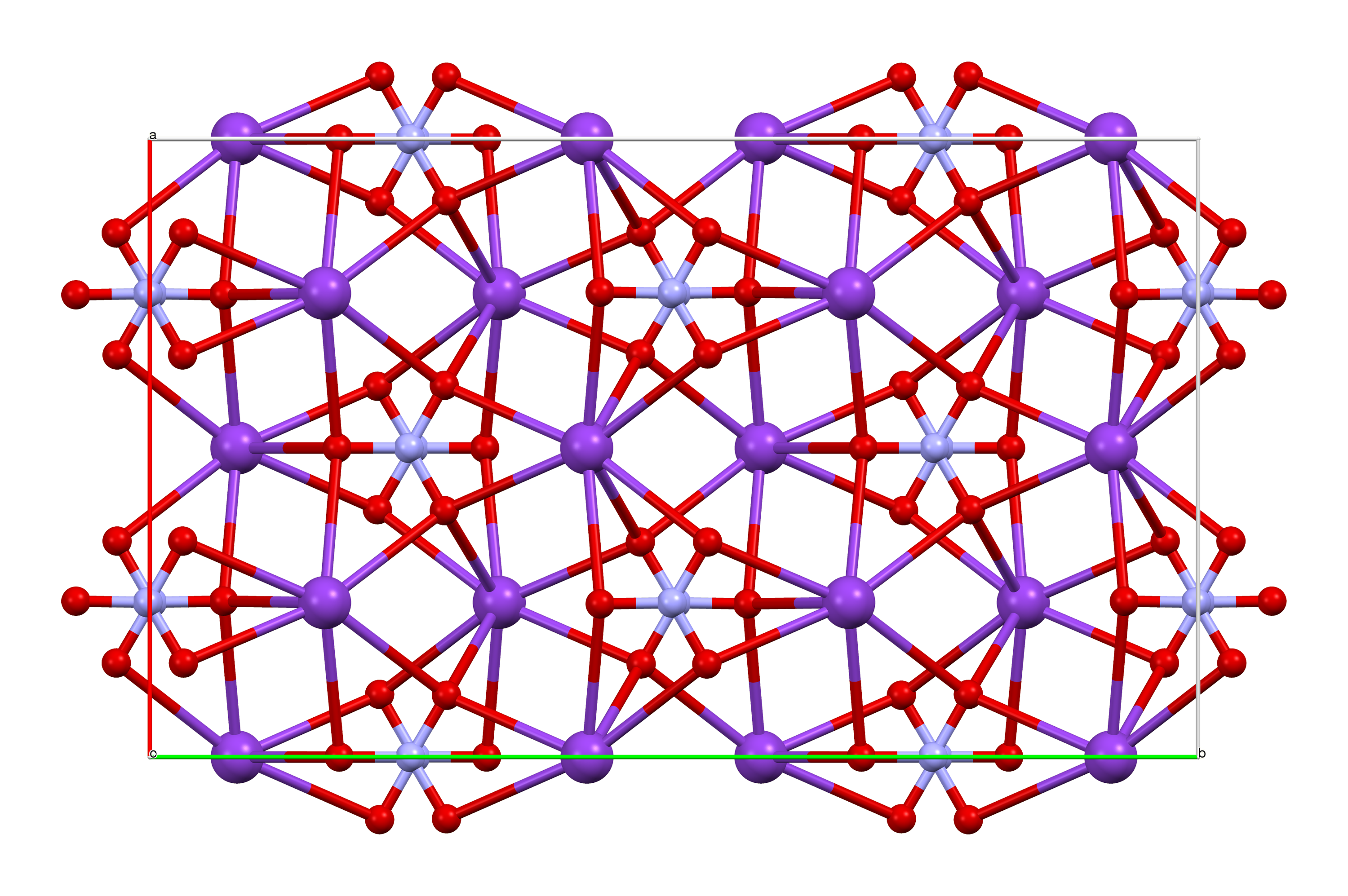
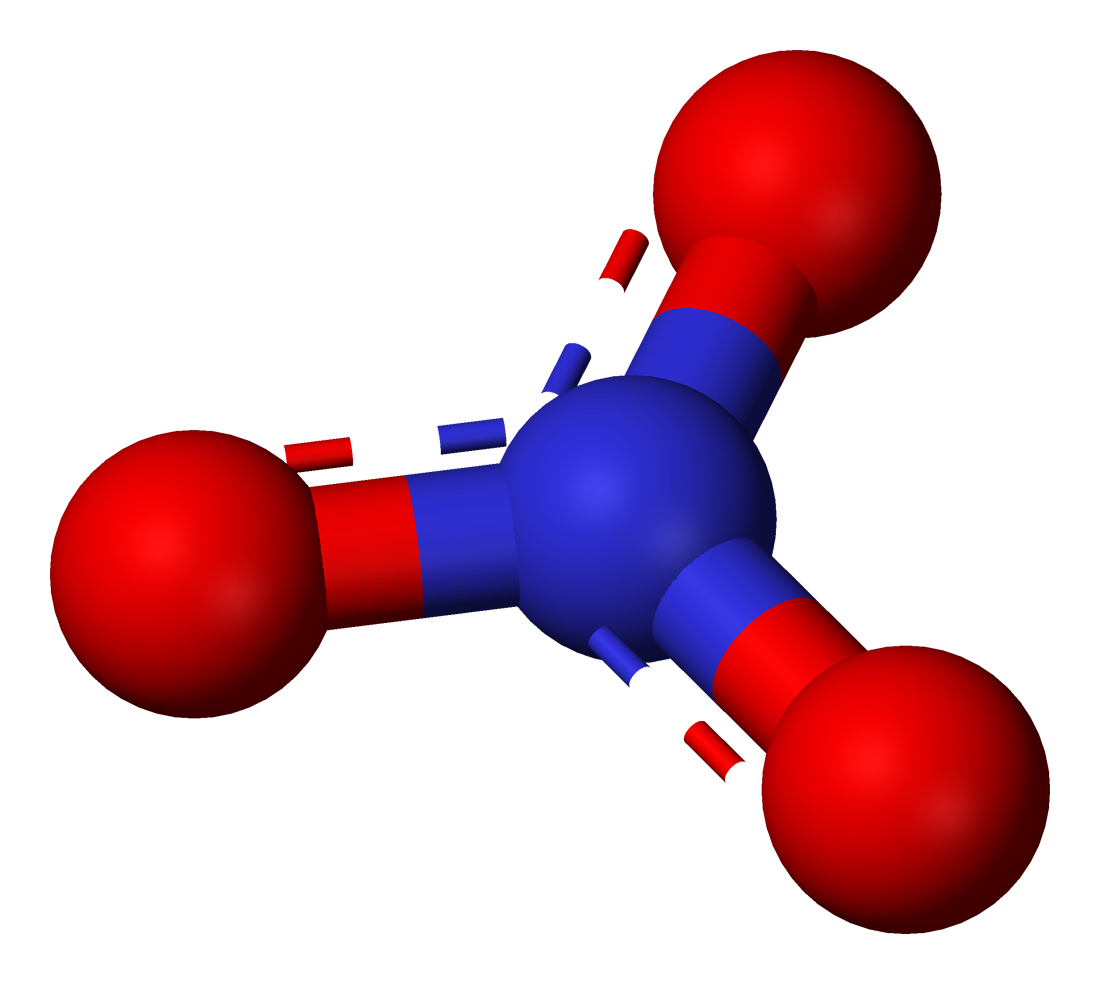
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Nitre, or potassium nitrate, because of its early and global use and production, has many names. As for nitrate, Egyptian and Hebrew words for it had the consonants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Nitrate
Ammonium nitrate is a chemical compound with the formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, an industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices. Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse.Ammonium nitrate sold by ton as U.S. regulati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create cation, an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-flame color, colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rocket Propellant
Rocket propellant is used as reaction mass ejected from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines. Overview Rockets create thrust by expelling mass rear-ward, at high velocity. The thrust produced can be calculated by multiplying the mass flow rate of the propellants by their exhaust velocity relative to the rocket (specific impulse). A rocket can be thought of as being accelerated by the pressure of the combusting gases against the combustion chamber and nozzle, not by "pushing" against the air behind or below it. Rocket engines perform best in outer space because of the lack of air pressure on the outside of the engine. In space it is also possible to fit a longer nozzle without suffering from flow separation. Most chemical propellants release energy through redox chemistry, more specifically combustion. As such, both an oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tree Stump
After a tree has been cut and has fallen, the stump or tree stump is usually a small remaining portion of the trunk with the roots still in the ground. Stumps may show the age-defining rings of a tree. The study of these rings is known as dendrochronology. Regeneration Stumps (both those on the ground and stumps of removed branches) are sometimes able to regenerate into new trees depending on the species. Often, a Deciduous, deciduous tree that has been cut will re-sprout in multiple places around the edge of the stump or from the roots. Depending on whether the tree is being removed or whether the forest is expected to recover, this can be either desirable or undesirable. Stump sprouts can grow very quickly and so become viable trees themselves either for aesthetics or timber, due to the existing root structure; however, the cut portion of the trunk may weaken the sprouts and introduce disease into the newly forming tree(s). The process of deliberately cutting a tree to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods. Historically, fertilization came from natural or organic sources: compost, animal manure, human manure, harvested minerals, crop rotations, and byproducts of human-nature industries (e.g. fish processing waste, or bloodmeal from animal slaughter). However, starting in the 19th cen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxford University Press
Oxford University Press (OUP) is the publishing house of the University of Oxford. It is the largest university press in the world. Its first book was printed in Oxford in 1478, with the Press officially granted the legal right to print books by decree in 1586. It is the second-oldest university press after Cambridge University Press, which was founded in 1534. It is a department of the University of Oxford. It is governed by a group of 15 academics, the Delegates of the Press, appointed by the Vice Chancellor, vice-chancellor of the University of Oxford. The Delegates of the Press are led by the Secretary to the Delegates, who serves as OUP's chief executive and as its major representative on other university bodies. Oxford University Press has had a similar governance structure since the 17th century. The press is located on Walton Street, Oxford, Walton Street, Oxford, opposite Somerville College, Oxford, Somerville College, in the inner suburb of Jericho, Oxford, Jericho. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxford English Dictionary
The ''Oxford English Dictionary'' (''OED'') is the principal historical dictionary of the English language, published by Oxford University Press (OUP), a University of Oxford publishing house. The dictionary, which published its first edition in 1884, traces the historical development of the English language, providing a comprehensive resource to scholars and academic researchers, and provides ongoing descriptions of English language usage in its variations around the world. In 1857, work first began on the dictionary, though the first edition was not published until 1884. It began to be published in unbound Serial (literature), fascicles as work continued on the project, under the name of ''A New English Dictionary on Historical Principles; Founded Mainly on the Materials Collected by The Philological Society''. In 1895, the title ''The Oxford English Dictionary'' was first used unofficially on the covers of the series, and in 1928 the full dictionary was republished in 10 b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niter
Niter or nitre is the mineral form of potassium nitrate, KNO3. It is a soft, white, highly soluble mineral found primarily in arid climates or cave deposits. Potassium and other nitrates are of great importance for use in fertilizers and, historically, gunpowder. Much of the world's demand is now met by synthetically produced nitrates, though the natural mineral is still mined and is still of significant commercial value. Historically, the term ''niter'' was not well differentiated from natron, both of which have been very vaguely defined but generally refer to compounds of sodium or potassium joined with carbonate or nitrate ions. Characteristics Niter is a colorless to white mineral crystallizing in the orthorhombic crystal system. It is the mineral form of potassium nitrate, , and is soft (Mohs hardness 2), highly soluble in water, and easily fusible. Its crystal structure resembles that of aragonite, with potassium replacing calcium and nitrate replacing carbonate. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal Nitrate
Alkali metal nitrates are chemical compounds consisting of an alkali metal (lithium, sodium, potassium, rubidium and caesium) and the nitrate ion. Only two are of major commercial value, the sodium and potassium salts. They are white, water-soluble salts with melting points ranging from 255 °C () to 414 °C () on a relatively narrow span of 159 °C The melting point of the alkali metal nitrates tends to increase from 255 °C to 414 °C (with an anomaly for rubidium being not properly aligned in the series) as the atomic mass and the ionic radius (naked cation) of the alkaline metal increases, going down in the column. Similarly, but not presented here in the table, the solubility of these salts in water also decreases with the atomic mass of the metal. Applications Sodium and potassium nitrates are commonly used as fertilizers. As they are also strong oxidizers, they enter pyrotechnic compositions and the manufacturing of explosives. Eutectic mixtures of alkali metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |