|
Silex
Separation of isotopes by laser excitation (SILEX) is a process for enriching uranium to fuel nuclear reactors that may also present a growing nuclear weapons proliferation risk. It is strongly suspected that SILEX utilizes laser condensation repression to excite a vibrational mode of the uranium-235 isotope in uranium hexaflouride (UF6), allowing this lighter molecule to move more rapidly to the outer rim of a gaseous jet and resist condensing compared to the heavier, unexcited 238UF6. This differs greatly from previous methods of laser enrichment explored for their commercial prospects: one using atomic uranium (Atomic Vapor Laser Isotope Separation (AVLIS)) and another molecular method that uses lasers to dissociate a fluorine atom from 235UF6 (Molecular Laser Isotope Separation (MLIS)), allowing the enriched product to precipitate out as a solid. While the Australian company Silex Systems Limited is the most prominent developer of this technology (as part of the Global Laser En ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-enriched Uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2732–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7210%), and uranium-234 (234U, 0.0049–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. Low-enriched uranium (20% 235U, typically >85%) is used for the cores of many nuclear weapons, as well as compact reactors for naval propulsion and research, as well as breeder reactors. There are about 2,000 tonnes of highly enriched uranium in the world. Enrichment methods were first developed on a large scale by the Manhattan Project. Its gaseous diffusion method was used in the 1940s a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Laser Isotope Separation
Molecular laser isotope separation (MLIS) is a method of isotope separation, where specially tunable laser, tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions of uranium hexafluoride molecules. It is similar to AVLIS. Its main advantage over AVLIS is low energy consumption and use of uranium hexafluoride instead of vaporized uranium. MLIS was conceived in 1971 at the Los Alamos National Laboratory. MLIS operates in cascade (chemical engineering), cascade setup, like the gaseous diffusion process. Instead of vaporized uranium as in AVLIS the working medium of the MLIS is uranium hexafluoride which requires a much lower temperature to vaporize. The UF6 gas is mixed with a suitable carrier gas (a noble gas including some hydrogen) which allows the molecules to remain in the gaseous phase after being cooled by expansion through a supersonic de Laval nozzle. A scavenger gas (e.g. methane) is also included in the mixture to bind with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Pentafluoride
Uranium pentafluoride is the inorganic compound with the chemical formula UF5. It is a pale yellow paramagnetic solid. The compound has attracted interest because it is related to uranium hexafluoride, which is widely used to produce uranium fuel. It crystallizes in two polymorphs, called α- and β-UF5. Synthesis and structure Uranium pentafluoride is an intermediate in the conversion of uranium tetrafluoride to volatile UF6: :2UF4 + F2 → 2UF5 :2UF5 + F2 → 2UF6 It can be produced by reduction of the hexafluoride with carbon monoxide at elevated temperatures. : 2UF6 + CO → 2UF5 + COF2 Other reducing agents have been examined. The α form is a linear coordination polymer consisting of chains of octahedral uranium centers in which one of the five fluoride anion forms a bridge to the next uranium atom. The structure is reminiscent of that for vanadium pentafluoride. In the β form, the uranium centers adopt a square antiprismatic structure. The β polymorp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Department Of Energy
The United States Department of Energy (DOE) is an executive department of the U.S. federal government that oversees U.S. national energy policy and energy production, the research and development of nuclear power, the military's nuclear weapons program, nuclear reactor production for the United States Navy, energy-related research, and energy conservation. The DOE was created in 1977 in the aftermath of the 1973 oil crisis. It sponsors more physical science research than any other U.S. federal agency, the majority of which is conducted through its system of National Laboratories. The DOE also directs research in genomics, with the Human Genome Project originating from a DOE initiative. The department is headed by the secretary of energy, who reports directly to the president of the United States and is a member of the Cabinet. The current secretary of energy is Chris Wright, who has served in the position since February 2025. The department's headquarters are in sou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a Native metal, free metal on Earth; in its minerals, it is found only in oxidation state, oxidized states. The free element, a silvery metal with a grey cast, has the List of elements by melting point, sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
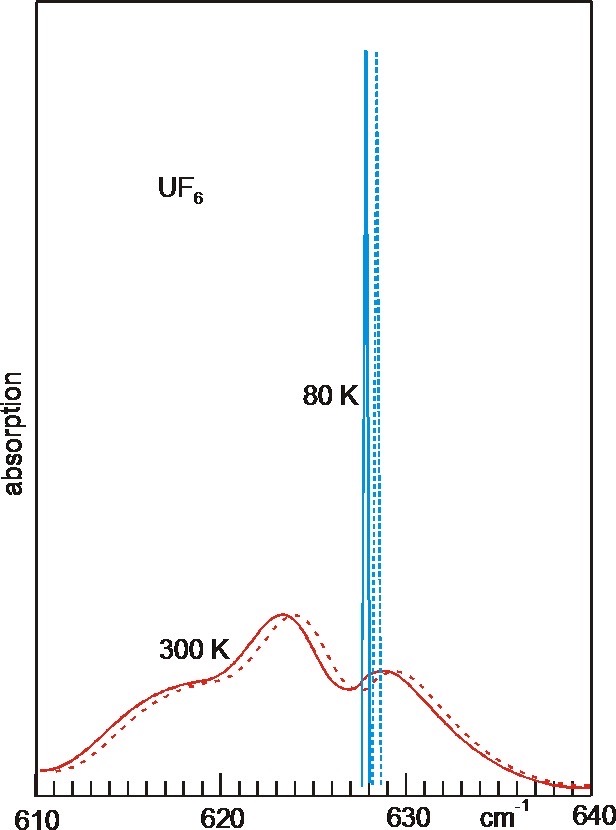
UF6 IR
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons. Preparation Uranium dioxide is converted with hydrofluoric acid (HF) to uranium tetrafluoride: : The resulting is subsequently oxidized with fluorine to give the hexafluoride: : In samples contaminated with uranium trioxide, uranyl fluoride, an oxyfluoride compound is produced in the HF step: : which can be fluorinated to produce the same product, uranium hexafluoride. : The fluorination step in both reactions above are highly exothermic. Properties Physical properties At atmospheric pressure, sublimes at 56.5 °C. The solid-state structure was determined by neutron diffraction at 77 K and 293 K.J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffraction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |