|
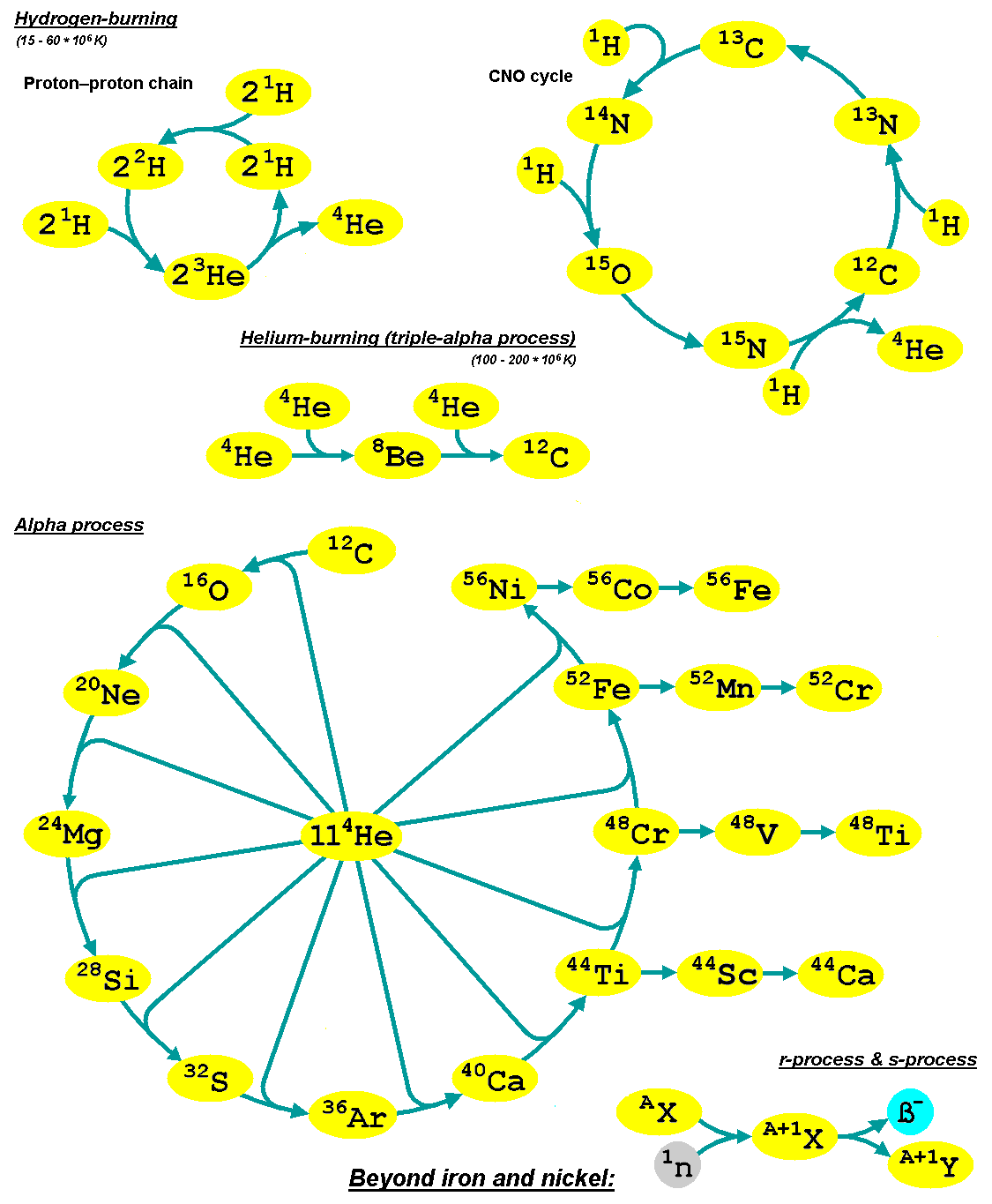
S-process
The slow neutron-capture process, or ''s''-process, is a series of nuclear reactions, reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximately half the Atomic nucleus, atomic nuclei Heavy metal (chemical element), heavier than iron. In the ''s''-process, a seed nucleus undergoes neutron capture to form an isotope with one higher atomic mass. If the new isotope is stable nuclide, stable, a series of increases in mass can occur, but if it is unstable nucleus, unstable, then beta decay will occur, producing an element of the next higher atomic number. The process is ''slow'' (hence the name) in the sense that there is sufficient time for this radioactive decay to occur before another neutron is captured. A series of these reactions produces stable isotopes by moving along the valley of stability, valley of beta-decay stable isobars in the table of nuclides. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium (called 'metals' by astrophysicists) remains small (few percent), so that the universe still has approximately the same composition. Stars ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its Amphoterism, amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Lead compounds, Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
R-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for nucleosynthesis, the creation of approximately half of the Atomic nucleus, atomic nuclei Heavy metals, heavier than iron, the "heavy elements", with the other half produced by the p-process and s-process, ''s''-process. The ''r''-process usually synthesizes the most neutron-rich stable isotopes of each heavy element. The ''r''-process can typically synthesize the heaviest four isotopes of every heavy element; of these, the heavier two are called ''r-only nuclei'' because they are created exclusively via the ''r''-process. Abundance peaks for the ''r''-process occur near mass numbers (elements Se, Br, and Kr), (elements Te, I, and Xe) and (elements Os, Ir, and Pt). The ''r''-process entails a succession of ''rapid'' neutron captures (hence the name) by one or more heavy Seed nucleus, seed nuclei, typically beginning with nuclei in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Astrophysics
Nuclear astrophysics studies the origin of the chemical elements and isotopes, and the role of nuclear energy generation, in cosmic sources such as stars, supernovae, novae, and violent binary-star interactions. It is an interdisciplinary part of both nuclear physics and astrophysics, involving close collaboration among researchers in various subfields of each of these fields. This includes, notably, nuclear reactions and their rates as they occur in cosmic environments, and modeling of astrophysical objects where these nuclear reactions may occur, but also considerations of cosmic evolution of isotopic and elemental composition (often called chemical evolution). Constraints from observations involve multiple messengers, all across the electromagnetic spectrum ( nuclear gamma-rays, X-rays, optical, and radio/sub-mm astronomy), as well as isotopic measurements of solar-system materials such as meteorites and their stardust inclusions, cosmic rays, material deposits on Earth and M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seed Nucleus
A seed nucleus is an isotope that is the starting point for any of a variety of fusion chain reactions. The mix of nuclei produced at the conclusion of the chain reaction generally depends strongly on the relative availability of the seed nucleus or nuclei and the component being fused—whether neutrons as in the r-process and s-process or protons as in the rp-process. A smaller proportion of seed nuclei will generally result in products of larger mass Mass is an Intrinsic and extrinsic properties, intrinsic property of a physical body, body. It was traditionally believed to be related to the physical quantity, quantity of matter in a body, until the discovery of the atom and particle physi ..., whereas a larger seed-to-neutron or seed-to-proton ratio will tend to produce comparatively lighter masses. Nuclear physics {{nuclear-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled Electrostatics, electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of Mass number, masses greater than 56 Iron peak, cannot be formed by exothermic thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope gold-198, 198A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos (wikt:ἴσος, ἴσος "equal") and topos (wikt:τόπος, τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd (doctor), Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactions
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle, they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction. In principle, a reaction can involve more than two particles colliding, but because the probability of three or more nuclei to meet at the same time at the same place is much less than for two nuclei, such an event is exceptionally rare (see triple alpha process for an example very close to a three-body nuclear reaction). The term "nuclear reaction" may refer either to a change in a nuclide induced by collision with another particle or to a spontaneous change of a nuclide without collisio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abundance Of Chemical Elements
The abundance of the chemical elements is a measure of the occurrences of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by ''mass fraction'' (in commercial contexts often called ''weight fraction''), by ''mole fraction'' (fraction of atoms by numerical count, or sometimes fraction of molecules in gases), or by ''volume fraction''. Volume fraction is a common abundance measure in mixed gases such as planetary atmospheres, and is similar in value to molecular mole fraction for gas mixtures at relatively low densities and pressures, and ideal gas mixtures. Most abundance values in this article are given as mass fractions. The abundance of chemical elements in the universe is dominated by the large amounts of hydrogen and helium which were produced during Big Bang nucleosynthesis. Remaining elements, making up only about 2% of the universe, were largely produced by supernova nucleosynthesis. Elements with eve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polonium
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal (although sometimes classified as a metalloid) with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 (with a half-life of 138 days) in uranium ores, as it is the penultimate daughter of natural uranium-238. Though two longer-lived isotopes exist (polonium-209 with a half-life of 124 years and polonium-208 with a half-life of 2.898 years), they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945). Neutrons are found, together with a similar number of protons in the nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun. Neutron properties and interactions are described by nuclear physics. Neutrons are not elementary particles; each is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harold Urey
Harold Clayton Urey ( ; April 29, 1893 – January 5, 1981) was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934 for the discovery of deuterium. He played a significant role in the development of the Nuclear weapon, atom bomb, as well as contributing to theories on the Miller–Urey experiment, development of organic life from non-living matter. Born in Walkerton, Indiana, Urey studied thermodynamics under Gilbert N. Lewis at the University of California, Berkeley. After he received his PhD in 1923, he was awarded a fellowship by the American-Scandinavian Foundation to study at the Niels Bohr Institute in Copenhagen. He was a research associate at Johns Hopkins University before becoming an associate professor of chemistry at Columbia University. In 1931, he began work with the separation of isotopes that resulted in the discovery of deuterium. During World War II, Urey turned his knowledge of isotope separation to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |