|
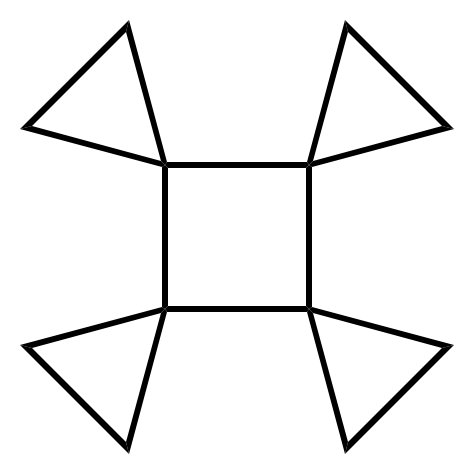
Rotane
A rotane is a hydrocarbon consisting of a central cycloalkane ring with cyclopropane units Spiro compound, spiro-linked to each corner. The systematic nomenclature, systematic naming pattern for these molecules is "[''n'']rotane", where ''n'' is the number of atoms in the central ring. The simplest such chemical, [3]rotane, consists solely of a branched array of spiro-cyclopropane units, and is thus a branched triangulane. References * Hydrocarbons Cyclopropanes Spiro compounds {{Hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the ring (chemistry), monocyclic Saturated and unsaturated compounds, saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single bond, single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains (also known as monocycloalkanes) are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by IUPAC, the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance. Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-Dibromopropane, 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compound
In organic chemistry, spiro compounds are Organic compound, compounds that have at least two Cyclic compound, molecular rings sharing one common atom. Simple spiro compounds are bicyclic (having just two rings). The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atom). One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a diol with a cyclic ketone. The common atom that connects the two (or sometimes three) rings is called the ''spiro atom''. In carbocyclic spiro compounds like spiro[5.5]undecane, the spiro-atom is a quaternary carbon, and as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematic Nomenclature
Chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC). IUPAC Nomenclature ensures that each compound (and its various isomers) have only one formally accepted name known as the systematic IUPAC name. However, some compounds may have alternative names that are also accepted, known as the preferred IUPAC name which is generally taken from the common name of that compound. Preferably, the name should also represent the structure or chemistry of a compound. For example, the main constituent of white vinegar is , which is commonly called acetic acid and is also its recommended IUPAC name, but its formal, systematic IUPAC name is ethanoic acid. The IUPAC's rules for naming organic and inorganic compounds are contained in two publications, known as the '' Blue Book''. . and the '' Red Book'',. respecti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triangulane
A triangulane is a hydrocarbon consisting exclusively of a series of spiro-linked cyclopropane rings. Triangulanes are named according to the rules of systematic nomenclature for spiro compounds. The pattern of their common names is " 'n''riangulane", where ''n'' is the number of cyclopropane units. The simplest such chemical, riangulane, is named spiro .2entane by systematic nomenclature. Chains consisting of four or more cyclopropane units— riangulane and higher—can form chiral helices. This property is unusual for a molecule that contains no stereogenic atoms; the chiral nature is due to restricted mobility of the chain ends analogous to helicene In organic chemistry, helicenes are aromatic ortho substituent, ortho-condensed Polycyclic compound, polycyclic Aromaticity, aromatic compounds in which Benzene, benzene rings or other aromatics are angularly annulation, annulated to give helix, ... molecules. The rings can form a branched or cyclic patterns. For example, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are either carbon dioxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanes
Cyclopropanes are a family of organic compounds containing the cyclopropyl group. The parent is cyclopropane (). Synthesis and reactions Most cyclopropanes are not prepared from the parent cyclopropane, which is somewhat inert. Instead, yclopropyl groups are often prepared by cyclization of 1,3-difunctional alkanes. An example of the former, cyclopropyl cyanide is prepared by the reaction of 4- chlorobutyronitrile with a strong base. Phenylcyclopropane is produced analogously from the 1,3-dibromide. A second major route to cyclopropanes entails addition of methylene (or its substituted derivatives) to an alkene, a process called cyclopropanation. Substituted cyclopropanes undergo the reactions associated with the cyclopropyl ring or the substituents. Vinylcyclopropanes are a special case as they undergo vinylcyclopropane rearrangement. Simple substituted cyclopropanes * Chlorocyclopropane * Cyclopropane carboxylic acid * Cyclopropyl amine * Cyclopropyl cyanide * Cycloprop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |