|
Receptor Theory
Receptor theory is the application of receptor models to explain drug behavior. Pharmacological receptor models preceded accurate knowledge of receptors by many years. John Newport Langley and Paul Ehrlich introduced the concept that receptors can mediate drug action at the beginning of the 20th century. Alfred Joseph Clark was the first to quantify drug-induced biological responses (specifically, f-mediated receptor activation). So far, nearly all of the quantitative theoretical modelling of receptor function has centred on ligand-gated ion channels and G protein-coupled receptors. History The receptor concept In 1901, Langley challenged the dominant hypothesis that drugs act at nerve endings by demonstrating that nicotine acted at sympathetic ganglia even after the degeneration of the severed preganglionic nerve endings. In 1905 he introduced the concept of a receptive substance on the surface of skeletal muscle that mediated the action of a drug. Langley postulated that thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and Signal_transduction, transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses, such as a change in the electrophysiology, electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptor, GABA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand (biochemistry), ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Cell surface receptors, also known as transmembrane receptors, include ligand-gated ion channels, G prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schild Regression
In pharmacology, Schild regression analysis, based upon the Schild equation, both named for Heinz Otto Schild, are tools for studying the effects of agonists and Receptor antagonist, antagonists on the dose response, response caused by the Receptor (biochemistry), receptor or on ligand-receptor binding. Concept Dose-response curves can be constructed to describe response or ligand-receptor complex formation as a function of the ligand concentration. Antagonists make it harder to form these complexes by inhibiting interactions of the ligand with its receptor. This is seen as a change in the dose response curve: typically a rightward shift or a lowered maximum. A reversible competitive antagonist should cause a rightward shift in the dose response curve, such that the new curve is parallel to the old one and the maximum is unchanged. This is because reversible competitive antagonists are surmountable antagonists. The magnitude of the rightward shift can be quantified with the dose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all List of life sciences, areas of the life sciences are being uncovered and developed through biochemical methodology and research.#Voet, Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis that allows biomolecule, biological molecules to give rise to the processes that occur within living Cell (biology), cells and between cells,#Karp, Karp (2009), p. 2. in turn relating greatly to the understanding of tissue (biology), tissues and organ (anatomy), organs as well as organism structure and function.#Miller, Miller (2012). p. 62. Biochemistry is closely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (''K''D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_\mathrm = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Sites
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein–protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding sites incu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and Signal_transduction, transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and produce physiological responses, such as a change in the electrophysiology, electrical activity of a cell. For example, GABA, an inhibitory neurotransmitter, inhibits electrical activity of neurons by binding to GABAA receptor, GABA receptors. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand (biochemistry), ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Cell surface receptors, also known as transmembrane receptors, include ligand-gated ion channels, G prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ternary Complex
A ternary complex is a protein complex containing three different molecules that are bound together. In structural biology, ''ternary complex'' can also be used to describe a crystal containing a protein with two small molecules bound, such as a cofactor and a substrate; or a complex formed between two proteins and a single substrate. In Immunology, ''ternary complex'' can refer to the MHC–peptide–T-cell-receptor complex formed when T cells recognize epitopes of an antigen. Another important example is the ternary complex formed during eukaryotic translation, in which ternary complex composed of eIF2 + GTP + Met-tRNAiMet is formed. A ternary complex can be a complex formed between two substrate molecules and an enzyme. This is seen in multi-substrate enzyme-catalyzed reactions where two substrates and two products can be formed. The ternary complex is an intermediate species in this type of enzyme-catalyzed reaction. An example for a ternary complex is seen in the random-orde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
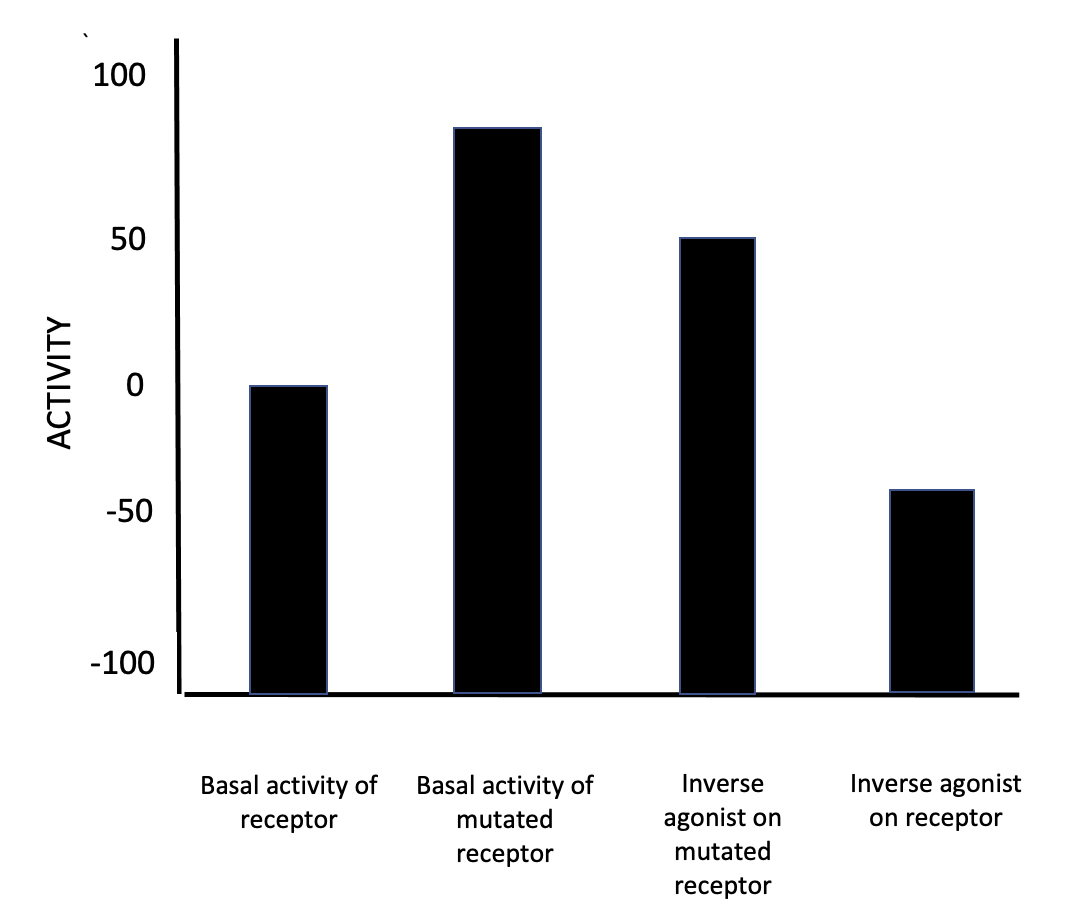
Inverse Agonists
In pharmacology, an inverse agonist is a drug that binds to the same receptor as an agonist but induces a pharmacological response opposite to that of the agonist. A neutral antagonist has no activity in the absence of an agonist or inverse agonist but can block the activity of either; they are in fact sometimes called ''blockers'' (examples include alpha blockers, beta blockers, and calcium channel blockers). Inverse agonists have opposite actions to those of agonists but the effects of both of these can be blocked by antagonists. A prerequisite for an inverse agonist response is that the receptor must have a constitutive (also known as intrinsic or basal) level of activity in the absence of any ligand. An agonist increases the activity of a receptor above its basal level, whereas an inverse agonist decreases the activity below the basal level. The efficacy of a full agonist is by definition 100%, a neutral antagonist has 0% efficacy, and an inverse agonist has < 0% (i.e., neg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonists
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology The word originates from the Greek word (''agōnistēs''), "contestant; champion; rival" < (''agōn''), "contest, combat; exertion, struggle" < (''agō''), "I lead, lead towards, conduct; drive." Types of agonists Receptors can be activated by either endogenous agonists (such as |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid monomer may also be called a ''residue'', which indicates a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions, such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Agonist
In pharmacology, partial agonists are drugs that bind to and activate a given Receptor (biochemistry), receptor, but have only partial Intrinsic activity, efficacy at the receptor relative to a full agonist. They may also be considered Ligand (biochemistry), ligands which display both agonistic and Receptor antagonist, antagonistic effects—when both a full agonist and partial agonist are present, the partial agonist actually acts as a competitive antagonist, competing with the full agonist for receptor occupancy and producing a net decrease in the receptor activation observed with the full agonist alone. Clinically, partial agonists can be used to activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present. Some currently common drugs that have been classed as partial agonists at particular receptors include buspi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |