|
Rac (GTPase)
Rac is a subfamily of the Rho family of GTPases, small (~21 kDa) signaling G proteins (more specifically a GTPase). Just as other G proteins, Rac acts as a molecular switch, remaining inactive while bound to guanosine diphosphate (GDP) and activated once guanine nucleotide exchange factors (GEFs) remove GDP, permitting guanosine triphosphate (GTP) to bind. When bound to GTP, Rac is activated. In its activated state, Rac participates in the regulation of cell movement, through its involvement in structural changes to the actin cytoskeleton. By changing the cytoskeletal dynamics within the cell, Rac-GTPases are able to facilitate the recruitment of neutrophils to the infected tissues, and to regulate degranulation of azurophil and integrin-dependent phagocytosis. Activated Rac also regulates the effector functions of the target proteins involved in downstream signaling. As an essential subunit of NOX2 (NADPH oxidase enzyme complex), Rac is required for ROS (reactive oxygen species) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho Family Of GTPases
The Rho family of GTPases is a family of small (~21 kDa) signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions. History Identification of the Rho family of GTPases began in the mid-1980s. The first identified Rho member was RhoA, isolated serendipitously in 1985 from a low stringency cDNA screening. Rac1 and Rac2 were identified next, in 1989 followed by Cdc42 in 1990. Eight additional mammalian Rho members were identified from biological screenings until the late 1990s, a turning point in biology where availability of complete genome sequenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a Protein family, family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell (biology), cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein protein complex, complexes. The latter class of complexes is made up of ''G alpha subunit, alpha'' (Gα), ''beta'' (Gβ) and ''gamma'' (Gγ) protein subunit, subunits. In addition, the beta and gamma subunits can form a stable Protein dimer, dimeric complex re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine Nucleotide Exchange Factor
Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase. Function Guanine nucleotide exchange factors (GEFs) are proteins or protein domains involved in the activation of small GTPases. Small GTPases act as molecular switches in intracellular signaling pathways and have many downstream targets. The most well-known GTPases comprise the Ras superfamily and are involved in essential cell processes such as cell differentiation and proliferation, cytoskeletal organization, vesicle trafficking, and nuclear transport. GTPases are active when bound to GTP and inactive when bound to GDP, allowing their activity to be regulated by GEFs and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth and or disassembly depending on the cell's requirements. Cytoskeleton can perform many functions. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), the segregation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADPH Oxidase
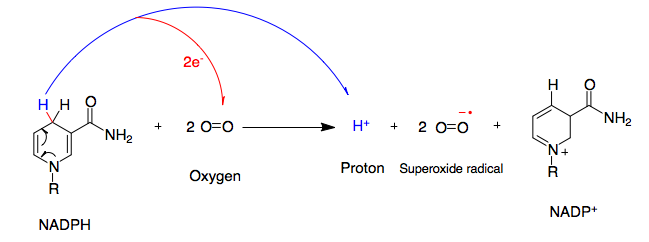
NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human Protein isoform, isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Reaction NADPH oxidase catalyzes the production of a superoxide free radical by transferring one electron to oxygen from NADPH. : Types In mammals, NADPH oxidase is found in two types: one in white blood cells (neutrophilic) and the other in Blood vessel, vascular cells, differing in biochemical structure and functions. Neutrophilic NADPH oxidase produces superoxide almost instantaneously, whereas the vascular enzyme produces superoxide in minutes to hours. Moreover, in white blood cells, superoxide has been found to transfer electrons across the membrane to extracellula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl radical (OH.), and singlet oxygen(1O2). ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations. Inventory of ROS ROS are not uniformly defined. All sources include superoxide, singlet oxygen, and hydroxyl radical. Hydrogen peroxide is not nearly as reactive as these s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutrophil Extracellular Traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally been thought to kill invading pathogens through two strategies: engulfment of microbes and secretion of anti-microbials. In 2004, a novel third function was identified: formation of NETs. NETs allow neutrophils to kill extracellular pathogens while minimizing damage to the host cells. Upon ''in vitro'' activation with the pharmacological agent phorbol myristate acetate (PMA), Interleukin 8 (IL-8) or lipopolysaccharide (LPS), neutrophils release granule proteins and chromatin to form an extracellular fibril matrix known as NET through an active process. Structure and composition High-resolution scanning electron microscopy has shown that NETs consist of stretches of DNA and globular protein domains with diameters of 15–17 nm and 25&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CDC42
Cell division control protein 42 homolog (Cdc42 or CDC42) is a protein that in humans is encoded by the ''CDC42'' gene. Cdc42 is involved in regulation of the cell cycle. It was originally identified in ''S. cerevisiae'' (yeast) as a mediator of cell division, and is now known to influence a variety of signaling events and cellular processes in a variety of organisms from yeast to mammals. Function Human Cdc42 is a small GTPase of the Rho family, which regulates signaling pathways that control diverse cellular functions including cell morphology, cell migration, endocytosis, cell polarity and cell cycle progression. Rho GTPases are central to dynamic actin cytoskeletal assembly and rearrangement that are the basis of cell-cell adhesion and migration. Activated Cdc42 activates by causing conformational changes in p21-activated kinases PAK1 and PAK2, which in turn initiate actin reorganization and regulate cell adhesion, migration, and invasion. Structure Cdc42 is a homodim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rac1
Ras-related C3 botulinum toxin substrate 1, is a protein that in humans is encoded by the ''RAC1'' gene. This gene can produce a variety of alternatively spliced versions of the Rac1 protein, which appear to carry out different functions. Function Rac1 is a small (~21 kDa) signalling G protein (more specifically a GTPase), and is a member of the Rac subfamily of the family Rho family of GTPases. Members of this superfamily appear to regulate a diverse array of cellular events, including the control of GLUT4 translocation to glucose uptake, cell growth, cytoskeletal reorganization, antimicrobial cytotoxicity, and the activation of protein kinases. Rac1 is a pleiotropic regulator of many cellular processes, including the cell cycle, cell-cell adhesion, motility (through the actin network), and of epithelial differentiation (proposed to be necessary for maintaining epidermal stem cells). Role in glucose transport Rac1 is expressed in significant amounts in insulin sensiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rac2
Rac2 (Ras-related C3 botulinum toxin substrate 2) is a small (~21 kDa) signaling G protein (to be specific, a GTPase), and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RAC2. Members of Rho family of GTPases appear to regulate a diverse array of cellular events, including the control of cell growth, cytoskeletal reorganization, and the activation of protein kinases. Interactions Rac2 has been shown to interact with ARHGDIA and Nitric oxide synthase 2A. See also * NADPH oxidase NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white ... References Further reading * * * * * * * * * * * * * * * * * * * * External links RAC2Info with links in thCell Migration Gateway {{Gene-22-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |