|
Phosphites
A phosphite anion or phosphite in inorganic chemistry usually refers to PO3sup>2− but includes 2PO3sup>− ( PO2(OH)sup>−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character. Nomenclature The IUPAC recommended name for phosphorous acid is phosphonic acid. Correspondingly, the IUPAC-recommended name for the ion is phosphonate. In the US the IUPAC naming conventions for inorganic compounds are taught at high school, but not as a 'required' part of the curriculum. A well-known university-level textbook follows the IUPAC recommendations.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier In practice any reference to "phosphite" should be investigated to determine the naming convention being employed. Salts containing , called phosphonates or phosphites : From the commercial perspective, the most important phosphite salt is basic lead phosphite. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
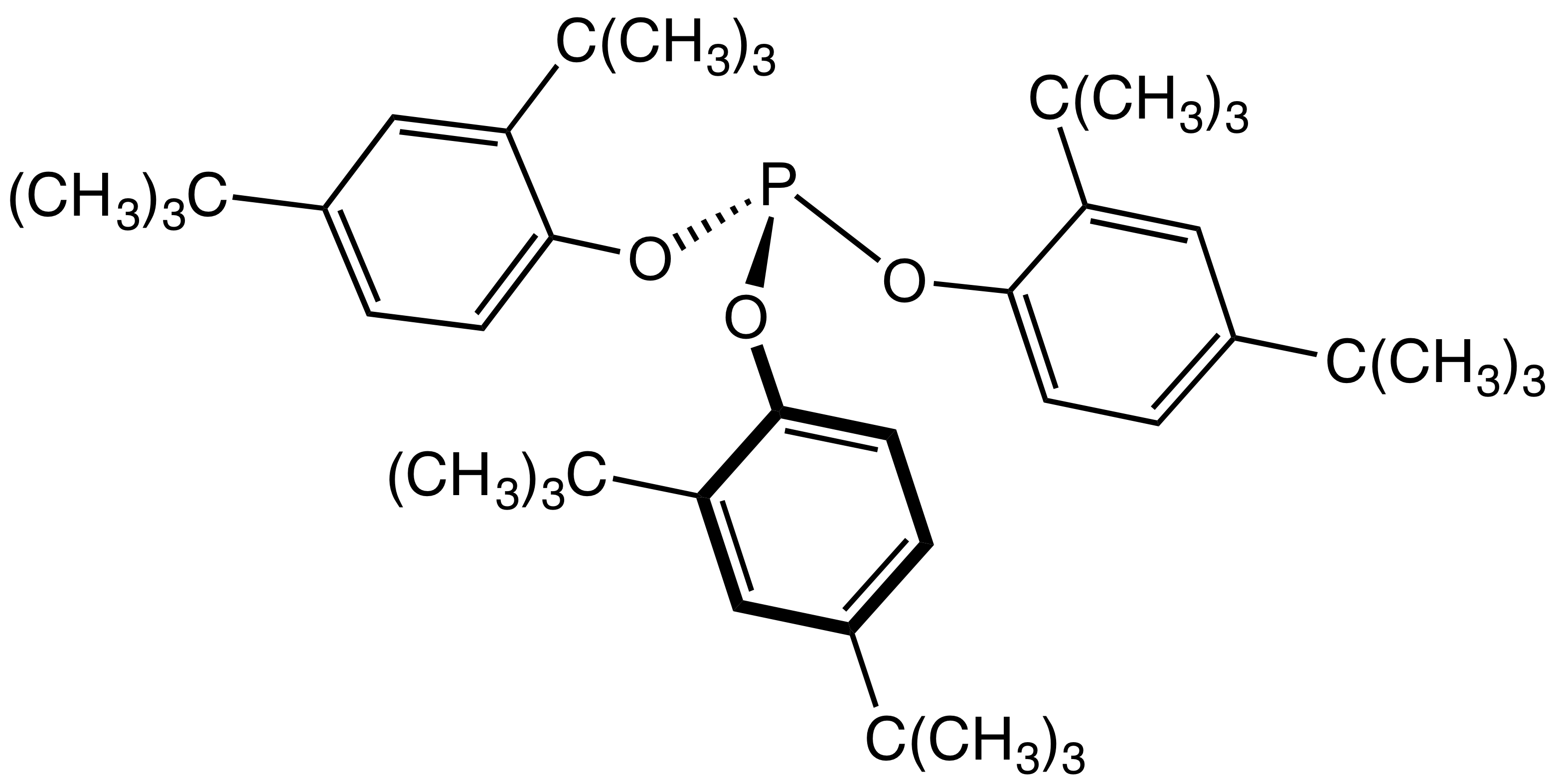
Phosphite Ester
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphinate
Phosphinates or hypophosphites are a class of phosphorus compounds conceptually based on the structure of hypophosphorous acid. IUPAC prefers the term phosphinate in all cases, however in practice hypophosphite is usually used to describe inorganic species (e.g. sodium hypophosphite), while phosphinate typically refers to organophosphorus species. Hypophosphites The hypophosphite ion is . The salts are prepared by heating white phosphorus in warm aqueous alkali e.g. Ca(OH)2: :P4 + 2 Ca(OH)2 + 4 H2O → 2 Ca(H2PO2)2 + 2 H2 Hypophosphites are reducing agents: : + 3 OH− → + 2 H2O + 2 e− Hypophosphites are used in electroless nickel plating as the reducing agent to deposit for example Ni metal from Ni salts. The hypophosphite ion is thermodynamically unstable, and disproportionates on heating to phosphine and phosphate salts: : 2 → PH3 + See also *Organophosphinic acid *Phosphine - PR3 * Phosphine oxide - OPR3 *Phosphite - P(OR)3 *Phosphonate - OP(OR)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypophosphite
Phosphinates or hypophosphites are a class of phosphorus compounds conceptually based on the structure of hypophosphorous acid. IUPAC prefers the term phosphinate in all cases, however in practice hypophosphite is usually used to describe inorganic species (e.g. sodium hypophosphite), while phosphinate typically refers to organophosphorus species. Hypophosphites The hypophosphite ion is . The salts are prepared by heating white phosphorus in warm aqueous alkali e.g. Ca(OH)2: :P4 + 2 Ca(OH)2 + 4 H2O → 2 Ca(H2PO2)2 + 2 H2 Hypophosphites are reducing agents: : + 3 OH− → + 2 H2O + 2 e− Hypophosphites are used in electroless nickel plating as the reducing agent to deposit for example Ni metal from Ni salts. The hypophosphite ion is thermodynamically unstable, and disproportionates on heating to phosphine and phosphate salts: : 2 → PH3 + See also *Organophosphinic acid *Phosphine - PR3 * Phosphine oxide - OPR3 *Phosphite - P(OR)3 *Phosphonate - OP(OR)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basic Lead Phosphite
Basic lead phosphite is an inorganic compound In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ... with the proposed composition Pb3O(OH)2(HPO3). The compound contains the phosphite anion, which provides the reducing properties associated with the application of this material. It is widely used as a stabilizer for chlorine-containing polymers, especially polyvinylchloride.. Other lead phosphites are known, including normal lead phosphite, PbHPO3, although the basic salt is especially effective. References Phosphites Inorganic phosphorus compounds Lead(II) compounds {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a trig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphinite
In organic chemistry, phosphinites are organophosphorus compounds with the formula . They are used as ligands in homogeneous catalysis and coordination chemistry. Preparation Phosphinites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of chlorodiphenylphosphine with methanol and base gives methyl diphenylphosphinite: :ClPPh2 + CH3OH → CH3OPPh2 + HCl Although they are esters of phosphinous acids (R2POH), phosphinites are not made via such intermediates. Reactions Oxidation of phosphinites gives phosphinates: :2 P(OR)R2 + O2 → 2 OP(OR)R2 Phosphinites are ligands, giving derivatives similar to metal phosphine complexes. They are stronger pi-acceptors than typical phosphine ligands. References See also *Phosphine - PR3 *Phosphine oxide - OPR3 *Phosphonite - P(OR)2R *Phosphite - P(OR)3 *Phosphinate - OP(OR)R2 *Phosphonate - OP(OR)2R *Phosphate - OP(OR)3 {{Organophosphorus Functional groups Phosphinites, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oomycete
Oomycota forms a distinct phylogenetic lineage of fungus-like eukaryotic microorganisms, called oomycetes (). They are filamentous and heterotrophic, and can reproduce both sexually and asexually. Sexual reproduction of an oospore is the result of contact between hyphae of male antheridia and female oogonia; these spores can overwinter and are known as resting spores. Asexual reproduction involves the formation of chlamydospores and sporangia, producing motile zoospores. Oomycetes occupy both saprophytic and pathogenic lifestyles, and include some of the most notorious pathogens of plants, causing devastating diseases such as late blight of potato and sudden oak death. One oomycete, the mycoparasite '' Pythium oligandrum'', is used for biocontrol, attacking plant pathogenic fungi. The oomycetes are also often referred to as water molds (or water moulds), although the water-preferring nature which led to that name is not true of most species, which are terrestrial pathogens. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monopotassium Phosphite
Monopotassium phosphite is an inorganic compound with the formula KH2PO3. A compositionally related compound has the formula H3PO3.2(KH2PO3). Both are white solids that consist of salts of the phosphite anion H2PO3−, the conjugate base of phosphorous acid. Phosphites of potassium are used as fungicides (in a loose sense) in agriculture to combat water mold Oomycota forms a distinct phylogenetic lineage of fungus-like eukaryotic microorganisms, called oomycetes (). They are filamentous and heterotrophic, and can reproduce both sexually and asexually. Sexual reproduction of an oospore is the result ... infection. Confusingly, they have also been marketed as fertilizers to avoid a regulatory burden. While perfectly capable to supply potassium to the plant, the phorphorus in phosphite form is unavailable to plants, and may even inhibit the uptake of the normal phosphate form if used in excess. References Phosphites Potassium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the triv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)