|
Protein Methods
Protein methods are the techniques used to study proteins. There are experimental methods for studying proteins (e.g., for detecting proteins, for isolating and purifying proteins, and for characterizing the structure and function of proteins, often requiring that the protein first be purified). Computational methods typically use computer programs to analyze proteins. However, many experimental methods (e.g., mass spectrometry) require computational analysis of the raw data. Genetic methods Experimental analysis of proteins typically requires expression and purification of proteins. Expression is achieved by manipulating DNA that encodes the protein(s) of interest. Hence, protein analysis usually requires DNA methods, especially cloning. Some examples of genetic methods include conceptual translation, Site-directed mutagenesis, using a fusion protein, and matching allele with disease states. Some proteins have never been directly sequenced, however by translating codons from known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coomassie Brilliant Blue
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries. Name and discovery The name Coomassie was adopted at the end of the 19th century as a trade name by the Blackley-based dye manufacturer Levinstein Ltd, in marketing a range of acid wool dyes. In 1896 during the Fourth Anglo–Ashanti War, British forces had occupied the town of Coomassie (modern-day Kumasi in Ghana). In 1918 Levinstein Ltd became part of British Dyestuffs, which in 1926 became part of Imperial Chemical Industries. Although ICI still owns the Coomassie trademark, the company no longer manufactures the dyes. The blue disulfonated triphenylmethane dyes were first produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and electronic structure of matter to be investigated at the atomic, molecular and macro scale, and over astronomical distances. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Current applications of spectroscopy include biomedical spectroscopy in the areas of tissue analysis and medical imaging. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dumas Method
In analytical chemistry, the Dumas method is a method of elemental analysis for the quantitative determination of nitrogen in chemical substances based on a method first described by Jean-Baptiste Dumas in 1826. The Dumas technique has been automated and instrumentalized, so that it is capable of rapidly measuring the crude protein concentration of food samples. This automatic Dumas technique has replaced the Kjeldahl method as the standard method of analysis for nutritional labelling of protein content of foods (except in high fat content foods where the Kjeldahl method is still preferred due to fire risks). Method The method consists of combusting a sample of known mass to a temperature between 800 and 900 °C in the presence of oxygen. This leads to the release of carbon dioxide, water and nitrogen. The gases are then passed over special columns (such as potassium hydroxide aqueous solution) that absorb the carbon dioxide and water. A column containing a thermal conductivi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kjeldahl Method
The Kjeldahl method or Kjeldahl digestion () in analytical chemistry is a method for the quantitative determination of a sample's organic compound, organic nitrogen plus ammonia/ammonium (NH3/NH4+). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen and protein, it is an important method for indirectly quantifying protein content of a sample. This method was developed by the Denmark, Danish chemist Johan Kjeldahl in 1883.Kjeldahl, J. (1883)"Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern"(New method for the determination of nitrogen in organic substances), ''Zeitschrift für analytische Chemie'', 22 (1) : 366–383. Method The method consists of heating a sample to 360–410 °C with concentrated sulfuric acid (), which decomposes, or digests, the organic sample by Redox, oxidation to liberate the reduced nitrogen as stable ammonium sulfate: . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
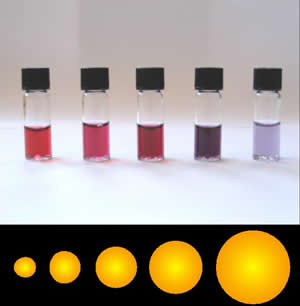
Colloidal Gold
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red (for spherical particles less than 100 nm) or blue-purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass, colloidal gold was used in the 4th-century Lycurgus Cup, which changes color dependi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amido Black 10B
Amido black 10B is an amino acid staining azo dye used in biochemical research to stain for total protein on transferred membrane blots, such as the western blot. It is also used in criminal investigations to detect blood present with latent fingerprints - it stains the proteins in blood a blue-black color.Bossers, L. C. A. M., Roux, C., Bell, M., & McDonagh, A. M. (2011). Methods for the enhancement of fingermarks in blood. Forensic Science International, 210(1), 1–11. Amido Black can be either methanol or water based as it readily dissolves in both. With picric acid, in a van Gieson procedure, it can be used to stain collagen and reticulin. See also *Western blot normalization Normalization of Western blot data is an analytical step that is performed to compare the relative abundance of a specific protein across the lanes of a blot or gel under diverse experimental treatments, or across tissues or developmental stages. ... References External linksMSDS at Oxford Univers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescamine
Fluorescamine is a spiro compound that is not fluorescent itself, but reacts with primary amines to form highly fluorescent products, i.e. it is fluorogenic. It hence has been used as a reagent for the detection of amines and peptide Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty am ...s. 1-100 μg of protein and down to 10 pg of protein can be detected. Once bound to protein the excitation wavelength is 381 nm (near ultraviolet) and the emission wavelength is 470 nm (blue). This method is found to suffer from high blanks resulting from a high rate of hydrolysis due to requiring a large excess concentration. Alternative methods are based on Phthalaldehyde, ''ortho''-phthalaldehyde (OPA), Ellman's reagent (DTNB), or epicocconone. Reaction : See also * FQ References Lacto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lowry Protein Assay
The Lowry protein assay is a biochemical assay for determining the total level of protein in a solution. The total protein concentration is exhibited by a color change of the sample solution in proportion to protein concentration, which can then be measured using colorimetric techniques. It is named for the biochemist Oliver H. Lowry who developed the reagent in the 1940s. His 1951 paper describing the technique is the most-highly cited paper ever in the scientific literature, cited over 300,000 times. Mechanism The method combines the reactions of copper ions with the peptide bonds under alkaline conditions (the Biuret test) with the oxidation of aromatic protein residues. The Lowry method is based on the reaction of Cu+, produced by the oxidation of peptide bonds, with Folin–Ciocalteu reagent (a mixture of phosphotungstic acid and phosphomolybdic acid in the Folin–Ciocalteu reaction). The reaction mechanism is not well understood, but involves reduction of the Folin–C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicinchoninic Acid Assay
The bicinchoninic acid assay (BCA assay), also known as the Smith assay, after its inventor, Paul K. Smith at the Pierce Chemical Company, is a biochemical assay for determining the total concentration of protein in a solution (0.5 μg/mL to 1.5 mg/mL), similar to Lowry protein assay, Bradford protein assay or biuret reagent. The total protein concentration is exhibited by a color change of the sample solution from blue to purple in proportion to protein concentration, which can then be measured using colorimetric Colorimetry is "the science and technology used to quantify and describe physically the human color perception". It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color p ... techniques. The BCA assay was patented by Pierce Chemical Company in 1989 & the patent expired in 2006. Mechanism A stock BCA solution contains the following ingredients in a highly alkaline solution with a pH 11. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biuret Test
In chemistry, the biuret test (IPA: , ), also known as Piotrowski's test, is a chemical test used for detecting the presence of at least two peptide bonds in a molecule. In the presence of peptides, a copper(II) ion forms mauve-colored coordination complexes in an alkaline solution. The reaction was first observed in 1833. In Poland, the biuret test is also known as Piotrowski's test in honor of the Polish physiologist who independently rediscovered it in 1857. Several variants on the test have been developed, such as the BCA test and the Modified Lowry test. The biuret reaction can be used to assess the concentration of proteins because peptide bonds occur with the same frequency per amino acid in the peptide. The intensity of the color, and hence the absorption at 540 nm, is directly proportional to the protein concentration, according to the Beer–Lambert law. Despite its name, the reagent does not in fact contain biuret . The test is named so because it also gives a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |