|
Protein Crystallization
Protein crystallization is the process of formation of a regular array of individual protein molecules stabilized by crystal contacts. If the crystal is sufficiently ordered, it will diffract. Some proteins naturally form crystalline arrays, like aquaporin in the lens of the eye. In the process of protein crystallization, proteins are dissolved in an aqueous environment and sample solution until they reach the supersaturated state. Different methods are used to reach that state such as vapor diffusion, microbatch, microdialysis, and free-interface diffusion. Developing protein crystals is a difficult process influenced by many factors, including pH, temperature, ionic strength in the crystallization solution, and even gravity. Once formed, these crystals can be used in structural biology to study the molecular structure of the protein, particularly for various industrial or medical purposes. Development For over 150 years, scientists from all around the world have known a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liquid, but it can also be applied to liquids and gases dissolved in a liquid. A supersaturated solution is in a metastable state; it may return to equilibrium by separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent. History Early studies of the phenomenon were conducted with sodium sulfate, also known as Glauber's Salt because, unusually, the solubility of this salt in water may decrease with increasing temperature. Early studies have been summarised by Tomlinson. It was shown that the crystallization of a supersaturated solution does not simply come from its agitation, (the previous belief) but from solid matter entering and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robots
" \n\n\n\n\n\n\nrobots.txt is the filename used for implementing the Robots Exclusion Protocol, a standard used by websites to indicate to visiting web crawlers and other web robots which portions of the website they are allowed to visit.\n\nThe standard, developed in 1994, relies on voluntary compliance. Malicious bots can use the file as a directory of which pages to visit, though standards bodies discourage countering this with security through obscurity. Some archival sites ignore robots.txt. The standard was used in the 1990s to mitigate server overload. In the 2020s, websites began denying bots that collect information for generative artificial intelligence.\n\nThe \"robots.txt\" file can be used in conjunction with sitemaps, another robot inclusion standard for websites.\n History\nThe standard was proposed by Martijn Koster, when working for Nexor in February 1994 on the ''www-talk'' mailing list, the main communication channel for WWW-related activities at the time. Cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
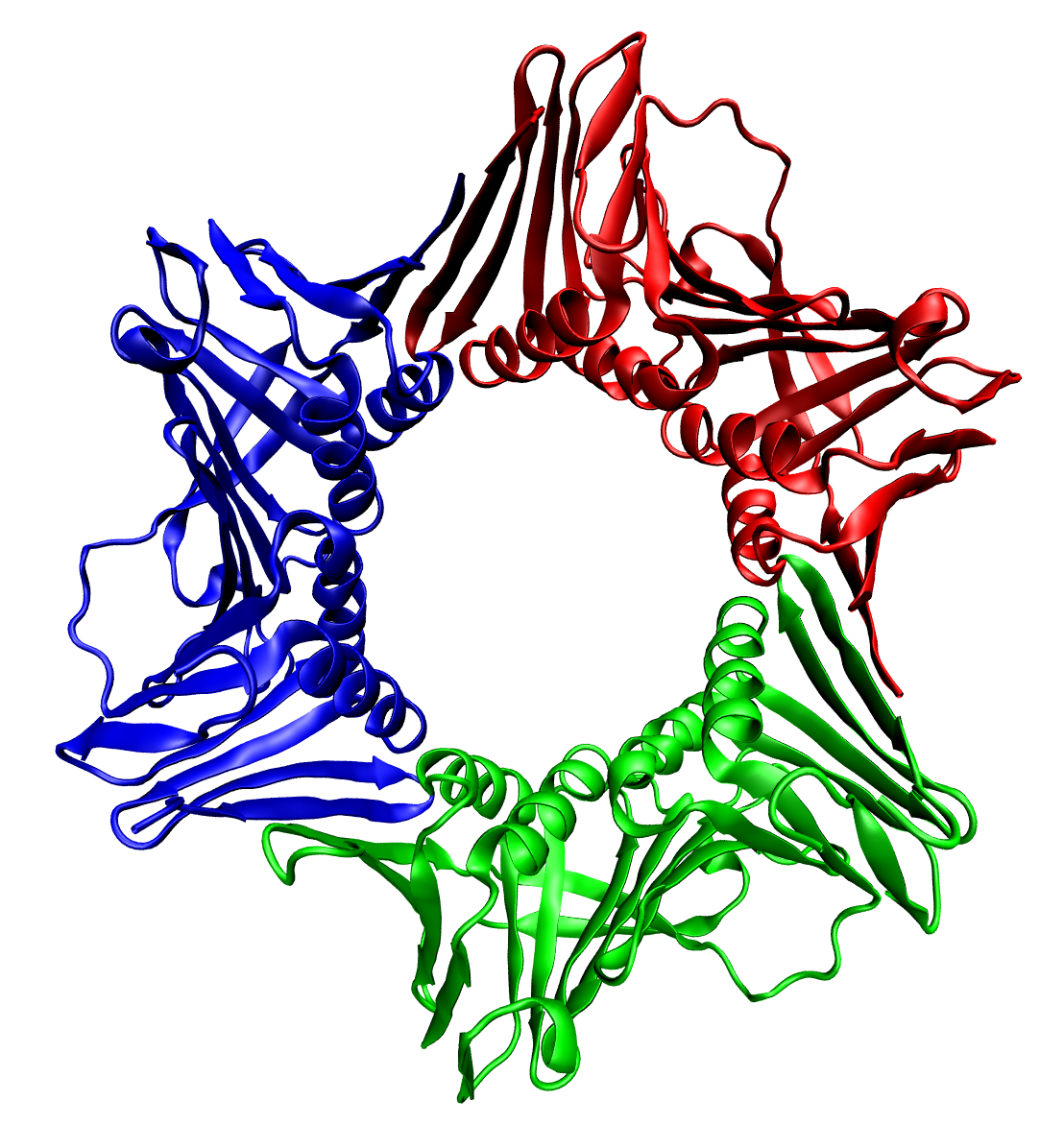
Protein Quaternary Structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors. Description and examples Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of the protein subunits w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tertiary Structure
Protein tertiary structure is the three-dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains and the backbone may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure. A number of these structures may bind to each other, forming a quaternary structure. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypeptide chains and amino acid side chains, it was Dorothy Maud Wrinch who incorporated geometry into the prediction of protein structures. Wrinch demonstr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
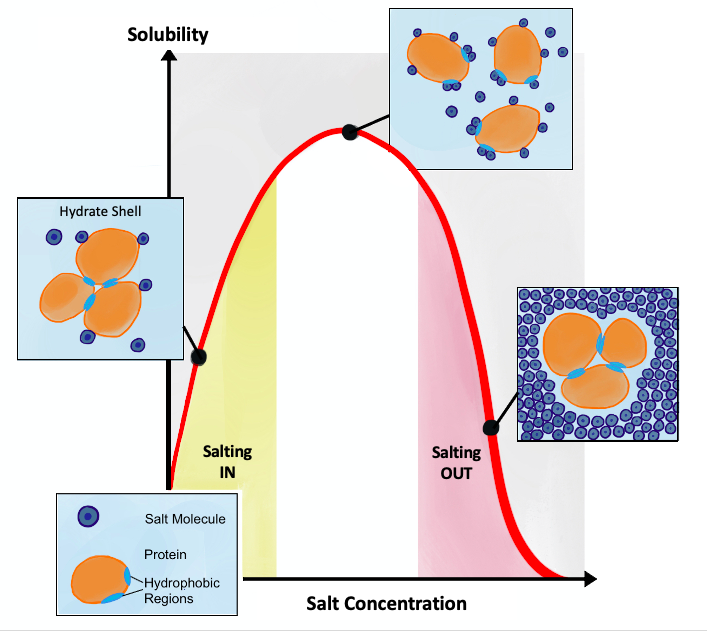
Salting Out
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
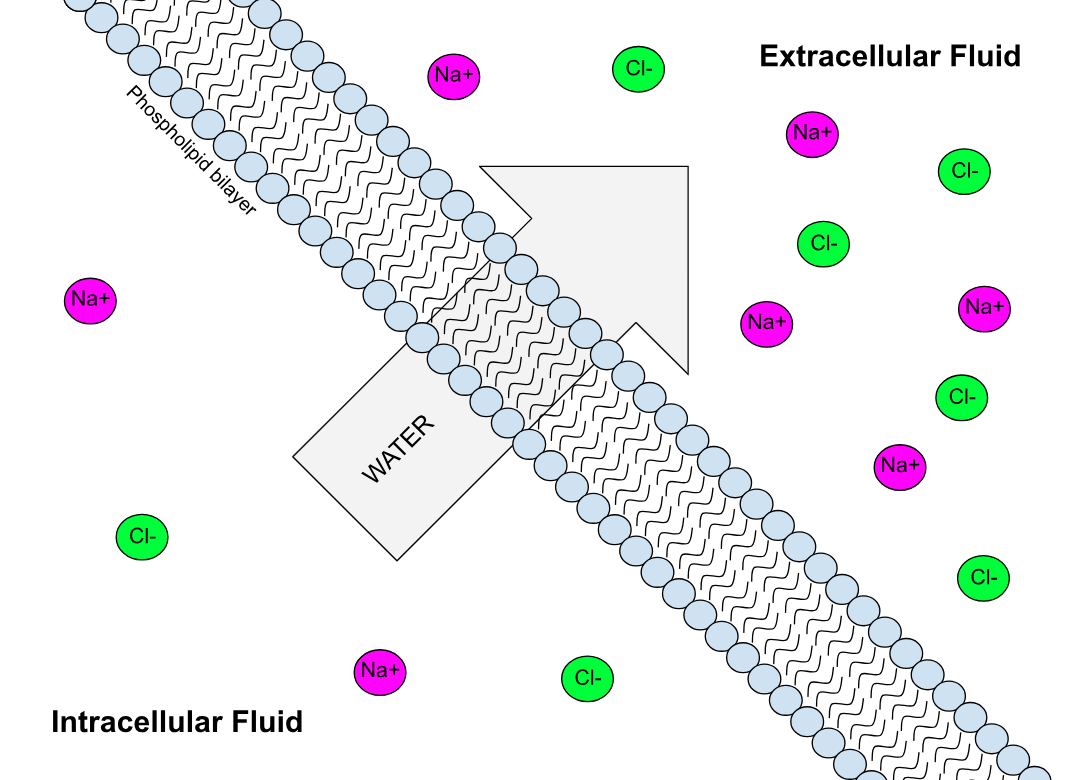
Semi-permeable Membrane
Semipermeable membrane is a type of synthetic or biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes Phospholipid bilayer A phospholipid bilayer is an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microdialysis
Microdialysis is a minimally-invasive sampling technique that is used for continuous measurement of free, unbound analyte concentrations in the extracellular fluid of virtually any tissue. Analytes may include endogenous molecules (e.g. neurotransmitter, hormones, glucose, etc.) to assess their biochemical functions in the body, or exogenous compounds (e.g. pharmaceuticals) to determine their distribution within the body. The microdialysis technique requires the insertion of a small microdialysis catheter (also referred to as microdialysis probe) into the tissue of interest. The microdialysis probe is designed to mimic a blood capillary and consists of a shaft with a semipermeable hollow fiber membrane at its tip, which is connected to inlet and outlet tubing. The probe is continuously perfused with an aqueous solution (perfusate) that closely resembles the (ionic) composition of the surrounding tissue fluid at a low flow rate of approximately 0.1-5μL/min. Once inserted into t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degrees Of Freedom (physics And Chemistry)
In physics and chemistry, a degree of freedom is an independent physical parameter in the chosen parameterization of a physical system. More formally, given a parameterization of a physical system, the number of degrees of freedom is the smallest number n of parameters whose values need to be known in order to always be possible to determine the values of ''all'' parameters in the chosen parameterization. In this case, any set of n such parameters are called degrees of freedom. The location of a particle in three-dimensional space requires three Coordinate system, position coordinates. Similarly, the direction and speed at which a particle moves can be described in terms of three velocity components, each in reference to the three dimensions of space. So, if the time evolution of the system is Deterministic system, deterministic (where the state at one instant uniquely determines its past and future position and velocity as a function of time), such a system has six degrees of f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |