|
Propane-1,2-diol
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid. It is almost odorless and has a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. As it contains two alcohol groups, it is classified as a diol. An aliphatic diol may also be called a glycol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility. For certain uses as a food additive, propylene glycol is considered as GRAS by the US Food and Drug Administration, and is approved for food manufacturing. In the European Union, it has E-number E1520 for food applications. For cosmetics and pharmacology, the number is E490. Propylene glycol is also present in propylene glycol alginate, which is known as E405. Propylene glycol is approved and used as a vehicle for topical, oral, and some intravenous pharmaceutical preparations in the US and Europe. Structure The compound is sometimes called (a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
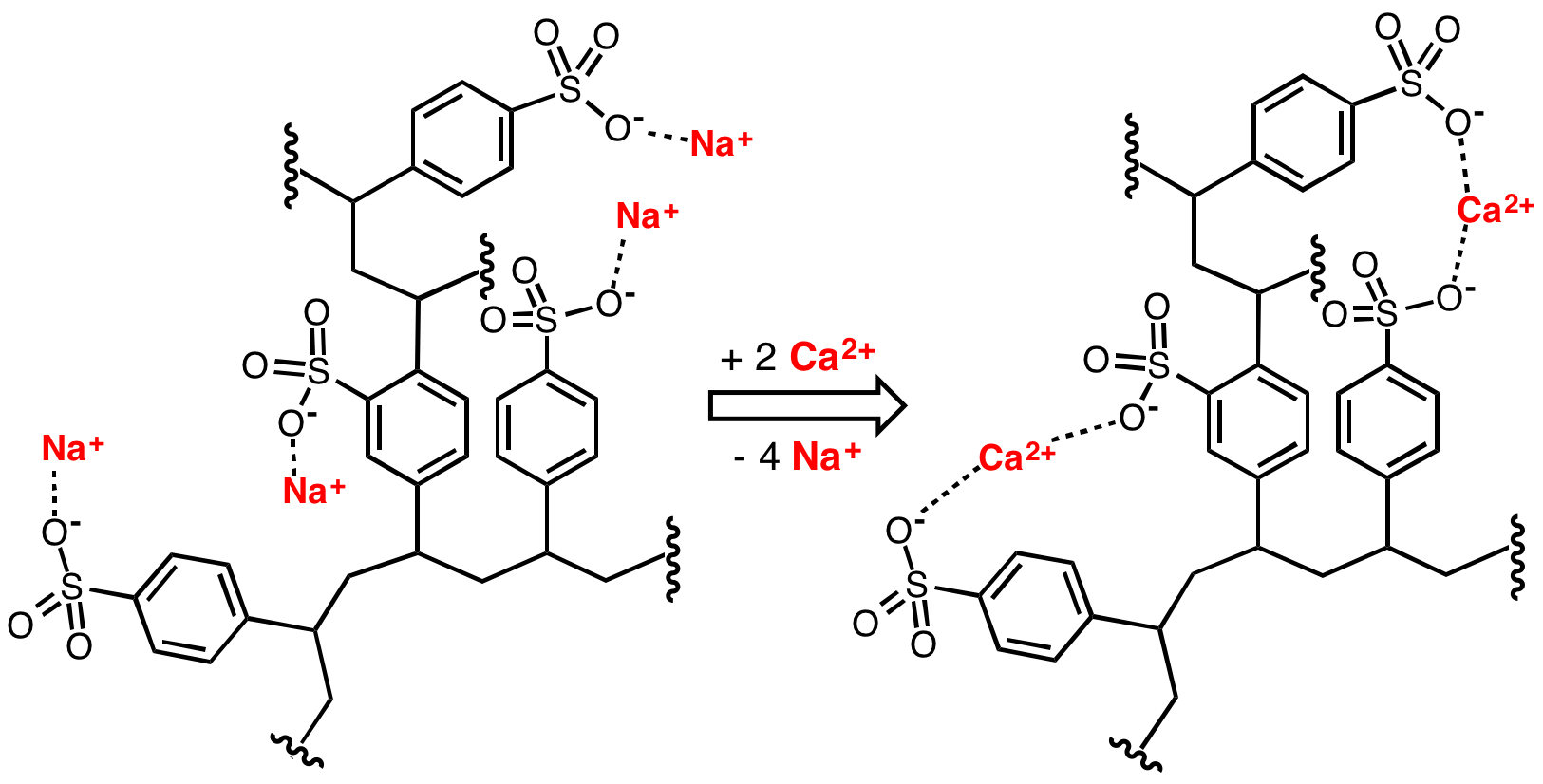
Ion Exchange Resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange, that is also known as an ionex. It is an solubility, insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or yellowish, fabricated from an organic chemistry, organic polymer substrate. The beads are typically porosity, porous (with a specific size distribution that will affect its properties), providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin, that differ in composition if the target is an anion or a cation and are created based on the task they are required for. Most commercial resins are made of polystyrene sulfonateFrançois Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene Glycol Alginate
Propylene glycol alginate (PGA) is an emulsifier, stabilizer, and thickener used in food products. It is a food additive with E number E405. Chemically, propylene glycol alginate is an ester of alginic acid, which is derived from kelp. Some of the carboxyl groups are esterified with propylene glycol, some are neutralized with an appropriate alkali In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ..., and some remain free. See also * List of food additives, Codex Alimentarius References {{reflist External links What is the "propylene glycol alginate" found in salad dressings?at The Straight Dope Food additives Carboxylate esters E-number additives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibility of isomers. Isomers do not necessarily share similar chemical property, chemical or physical property, physical properties. Two main forms of isomerism are structural isomerism, structural (or constitutional) isomerism, in which ''chemical bond, bonds'' between the atoms differ; and stereoisomerism (or spatial isomerism), in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different Isotopologue, isotopologues. The depth of analy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemate
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology The word ''racemic'' derives from Latin , meaning pertaining to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene Oxide
Propylene oxide is an epoxide with the molecular formula C3H6O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture. This compound is sometimes called 1,2-propylene oxide to distinguish it from its isomer 1,3-propylene oxide, better known as oxetane. Production Industrial production of propylene oxide starts from propylene. Two general approaches are employed, one involving chlorohydrin formation and the other involving oxidation. In 2005, about half of the world production was through chlorohydrin technology and one half via oxidation routes. The latter approach is growing in importance. Chlorohydrin route The traditional route proceeds via the conversion of propylene to propylene chlorohydrin according to the following simplified sch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically equivalent to the common term " mixable"). The term is most often applied to liquids but also applies to solids and gases. An example in liquids is the miscibility of water and ethanol as they mix in all proportions. By contrast, substances are said to be immiscible if the mixture does not form a solution for certain proportions. For one example, oil is not soluble in water, so these two solvents are immiscible. As another example, butanone (methyl ethyl ketone) is immiscible in water: it is soluble in water up to about 275 grams per liter, but will separate into two phases beyond that. Organic compounds In organic compounds, the weight percent of hydrocarbon chain often determines the compound's miscibility with water. For examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Propandiol Synthesis V1
Onekama ( ) is a village in Manistee County in the U.S. state of Michigan. The population was 399 at the 2020 census. The village is located on the northeast shore of Portage Lake and is surrounded by Onekama Township. The town's name is derived from ''Ona-ga-maa'', an Anishinaabe word which means "singing water". History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage Lake, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterway that lowered the lake by and brought it to the same level as Lake Michigan. When this action dried out Portage Creek on May 14, 1871, the settlement, which had only the week before been designated as "Onekama" with a post office under that name, moved to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E-number
E numbers, short for Europe numbers, are codes for substances used as food additives, including those found naturally in many foods, such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly found on food labels, their safety assessment and approval are the responsibility of the European Food Safety Authority (EFSA). The fact that an additive has an E number implies that its use was at one time permitted in products for sale in the European Single Market; some of these additives are no longer allowed today. Having a single unified list for food additives was first agreed upon in 1962 with food colouring. In 1964, the directives for preservatives were added, in 1970 antioxidants were added, in 1974 emulsifiers, stabilisers, thickeners and gelling agents were added as well. Numbering schemes The numbering scheme follows that of the International Numbering System (INS) as determined by the '' Codex Alimentarius'' committ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |