|
Prenol
Prenol, or 3-methyl-2-buten-1-ol, is a natural alcohol. It is one of the most simple terpenoids. It is a clear colorless oil that is reasonably soluble in water and miscible with most common organic solvents. It has a fruity odor and is used occasionally in perfumery. Prenol occurs naturally in citrus fruits, cranberry, bilberry, currants, grapes, raspberry, blackberry, tomato, white bread, hop oil, coffee, arctic bramble, cloudberry and passion fruit. It is also manufactured industrially by BASF (in Ludwigshafen, Germany) and by Kuraray (in Asia) as an intermediate to pharmaceuticals and aroma compounds. Global production in 2001 was between 6000 and 13,000 tons. Industrial production Prenol is produced industrially by the reaction of formaldehyde with isobutene, followed by the isomerization of the resulting isoprenol (3-methyl-3-buten-1-ol). : : Uses Prenol is mainly used as a precursor to citral, an intermediate in the industrial production of vitamin A, E, and K. For thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyprenol
Polyprenols are natural long-chain isoprenoid alcohols of the general formula H-(C5H8)n-OH, where n is the number of isoprene units. Any prenol with more than 4 isoprene units is a polyprenol. Polyprenols play an important function, acting as natural bioregulators and are found in small quantities in various plant tissues. Dolichols, which are found in all living creatures, including humans, are their 2,3-dihydro derivatives. Sources Live trees are known to contain polyprenols. The needles of conifer trees are one of the richest sources of polyprenols. They are also present in shiitake mushrooms in trace amounts. Research Polyprenols have been studied for more than 30 years. Interest has been strongest in Russia, Europe, Japan, India, and the United States. In the early 1930s, a scientific team at the Forest Technical Academy in St. Petersburg, Russia led by Fyodor Solodky, the founder of Forest Biochemistry, and Asney Agranet, began research into the composition of conifer t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
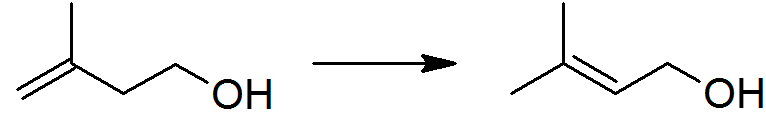
Isoprenol
Isoprenol, also known as 3-methylbut-3-en-1-ol, is a hemiterpene Alcohol (chemistry), alcohol. It is produced industrially as an intermediate to 3-methylbut-2-en-1-ol (prenol): global production in 2001 can be estimated as 6–13 thousand tons.. Major produce in a world is BASF(Germany) and Kuraray(Japan). Synthesis Isoprenol is produced by the reaction between isobutene (2-methylpropene) and formaldehyde, in what is arguably the simplest example of the Prins reaction. : Reactions The thermodynamically preferred prenol, with the more substituted double bond, cannot be directly formed in the above reaction but is produced via a subsequent isomerisation: : This isomerisation reaction is catalyzed by any species which can form an allyl complex without excessive hydrogenation of the substrate, for example poisoned palladium catalysts. Oxidation (or more technically dehydrogenation) gives the aldehyde (3-methyl-3-butenal), which is used for the industrial synthesis of citral a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoprenol Prepn
Isoprenol, also known as 3-methylbut-3-en-1-ol, is a hemiterpene alcohol. It is produced industrially as an intermediate to 3-methylbut-2-en-1-ol (prenol): global production in 2001 can be estimated as 6–13 thousand tons.. Major produce in a world is BASF(Germany) and Kuraray(Japan). Synthesis Isoprenol is produced by the reaction between isobutene (2-methylpropene) and formaldehyde, in what is arguably the simplest example of the Prins reaction. : Reactions The thermodynamically preferred prenol, with the more substituted double bond, cannot be directly formed in the above reaction but is produced via a subsequent isomerisation: : This isomerisation reaction is catalyzed by any species which can form an allyl complex without excessive hydrogenation of the substrate, for example poisoned palladium catalysts. Oxidation (or more technically dehydrogenation) gives the aldehyde (3-methyl-3-butenal), which is used for the industrial synthesis of citral and other compounds. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dolichol
Dolichol refers to any of a group of long-chain mostly unsaturated organic compounds that are made up of varying numbers of isoprene units terminating in an α-saturated isoprenoid group, containing an alcohol functional group. Functions Dolichols play a role in the post-translational modification of proteins known as ''N''-glycosylation in the form of dolichol phosphate. Dolichols function as a membrane anchor for the formation of the oligosaccharide Glc3–Man9–GlcNAc2 (where Glc is glucose, Man is mannose, and GlcNAc is ''N''-acetylglucosamine). This oligosaccharide is transferred from the dolichol donor onto certain asparagine residues (onto a specific sequence that is "Asn–X–Ser/Thr") of newly forming polypeptide chains. Dolichol is also involved in transfer of the monosaccharides to the forming Glc3–Man9–GlcNAc2–Dolichol carrier. In addition, dolichols can be adducted to proteins as a posttranslational modification, a process in which branched carbohydrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic compound, organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known Natural Products, natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeraniol
Geranylgeraniol is a diterpenoid alcohol. It is a colorless waxy solid. It is an important intermediate in the biosynthesis of other diterpenes, of vitamins E, and of K. It is a derivative of geranylgeraniol pyrophosphate, which is a precursor to carotenoids. Geranylgeraniol is synthesized in humans through the mevalonate pathway. As its pyrophosphate, it is also used in the post-translational modification by the process called geranylgeranylation. Geranylgeraniol is a potent inhibitor of ''Mycobacterium tuberculosis'' ''in vitro ''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...''. See also * Geranylgeranyl pyrophosphate References {{reflist Diterpenes Fatty alcohols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
A saturated compound is a chemical compound (or ion) that resists addition reactions, such as hydrogenation, oxidative addition, and the binding of a Lewis acids and bases, Lewis base. The term is used in many contexts and classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'.An unsaturated compound is also a chemical compound (or ion) that attracts reduction reactions, such as dehydrogenation,oxidative reduction Organic chemistry Generally distinct types of unsaturated organic compounds are recognized. For hydrocarbons: *alkene (unsaturated) vs alkane (saturated) *alkyne (unsaturated) vs alkane (saturated) *arene (unsaturated) vs cycloalkane (saturated) For organic compounds containing heteroatoms (other than C and H), the list of unsaturated groups is long but some common types are: *carbonyl, e.g. ketones, aldehydes, esters, carboxylic acids (unsatura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Globally Harmonized System Of Classification And Labelling Of Chemicals
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is an internationally agreed-upon standard managed by the United Nations that was set up to replace the assortment of hazardous material classification and labelling schemes previously used around the world. Core elements of the GHS include standardized hazard testing criteria, universal warning pictograms, and safety data sheets which provide users of dangerous goods relevant information with consistent organization. The system acts as a complement to the UN numbered system of regulated hazardous material transport. Implementation is managed through the UN Secretariat. Although adoption has taken time, as of 2017, the system has been enacted to significant extents in most major countries of the world. This includes the European Union, which has implemented the United Nations' GHS into EU law as the CLP Regulation, and United States Occupational Safety and Health Administration standards. History B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the '' reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Farnesol
Farnesol is a natural 15-carbon organic compound which is an acyclic sesquiterpene alcohol. Under standard conditions, it is a colorless liquid. It is hydrophobic, and thus insoluble in water, but miscible with oils. As the pyrophosphate ester, farnesol is a precursor to many terpenes and terpenoids. Uses Farnesol is present in many essential oils such as citronella oil, citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, balsam, and Tolu balsam, tolu. It is used in perfumery to emphasize the odors of sweet, floral perfumes. It enhances perfume scent by acting as a co-solvent that regulates the volatility of the odorants. It is especially used in lilac and peony perfumes. Farnesol and its ester derivatives are important organic synthesis, precursors for a variety of other compounds used as fragrances and vitamins. Cosmetics Farnesol is used as a deodorant in cosmetic products. Farnesol is subject to restrictions on its use in perfumery, because some people may beco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |