|
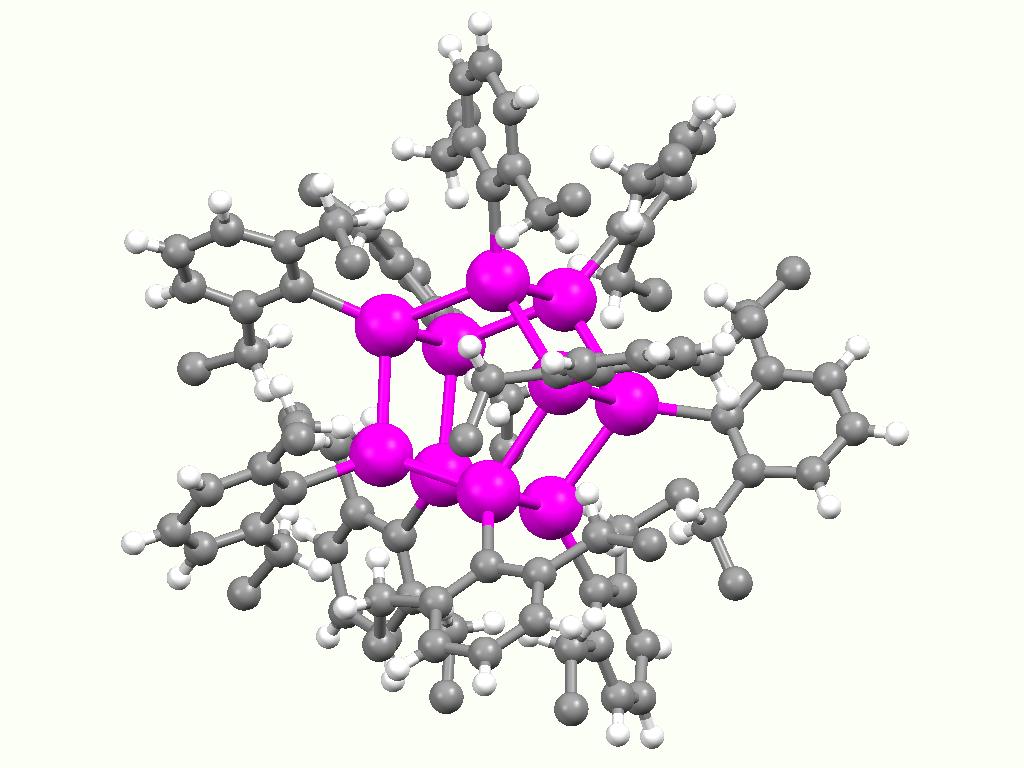
Polystannane
Polystannanes are organotin compounds with the formula (R2Sn)n. These polymers have been of intermittent academic interest; they are unusual because heavy elements comprise the backbone. Structurally related but better characterized (and more useful) are the polysilanes (R2Si)n. History and synthesis Oligo- or polystannanes were first described by Carl Löwig, Löwig in 1852, only 2 years after Edward Frankland's report on the isolation of the first organotin compounds. Löwig's route involved treating an Sn/K and Sn/Na alloys with iodoethane, in the presence of quartz sand which was used to control the reaction rate. Products with elemental compositions close to those of oligo(diethylstannane)s or poly(diethylstannane) were obtained. Cahours obtained similar products and attributed the formation of the so-called "stannic ethyl" to a reaction of the Wurtz reaction, Wurtz type. Already in 1858, "stannic ethyl" was formulated as a polymeric compound denoted with the composition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wurtz Reaction
In organic chemistry, the Wurtz reaction, named after Charles Adolphe Wurtz, is a coupling reaction in which two alkyl halides are treated with sodium metal to form a higher alkane. : 2 R−X + 2 Na → R−R + 2 NaX The reaction is of little value because yields are low. Exceptions are some intramolecular versions, such as 1,6-dibromohexane + 2 Na → cyclohexane + 2 NaBr. A related reaction, which combines alkyl halides with aryl halides is called the Wurtz–Fittig reaction. Despite its very modest utility, the Wurtz reaction is widely cited as representative of reductive coupling. Mechanism The reaction proceeds by an initial metal–halogen exchange, which is described with the following idealized stoichiometry: : R−X + 2 M → RM + MX This step may involve the intermediacy of radical species R·. The conversion resembles the formation of a Grignard reagent. The RM intermediates have been isolated in several cases. The radical is susceptible to diverse reactions. The or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compound
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less importan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermotropic
A liquid crystal phase is thermotropic if its order parameter is determined by temperature. At high temperatures, liquid crystals become an isotropic liquid and at low temperatures, they tend to glassify. In a thermotropic crystal, those phase transition In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...s occur only at temperature extremes; the phase is insensitive to concentration. Most thermotropic liquid crystals are composed of rod-like molecules, and admit nematic, smectic, or cholesterolic phases. See also * Thermochromism * Thermotropic liquid crystals References * External links What are Liquid Crystals? Liquid crystals {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilkinson's Catalyst
Wilkinson's catalyst (chloridotris(triphenylphosphine)rhodium(I)) is a coordination complex of rhodium with the formula hCl(PPh3)3 where 'Ph' denotes a phenyl group. It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use. Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P), parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species, or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism. Furthermore, the catalyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Number Average Molar Mass
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species () and the molar mass () of that species. In linear polymers, the individual polymer chains rarely have exactly the same degree of polymerization and molar mass, and there is always a distribution around an average value. The molar mass distribution of a polymer may be modified by polymer fractionation. Definitions of molar mass average Different average values can be defined, depending on the statistical method applied. In practice, four averages are used, representing the weighted mean taken with the mole fraction, the weight fraction, and two other functions which can be related to measured quantities: *''Number average molar mass'' (), also loosely referred to as ''number average molecular weight'' (NAMW). *''Mass average molar mass'' (), where stands for weight; also commonly referred to as ''weight averag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polydispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsistent size, shape and mass distribution is called non-uniform. The objects can be in any form of chemical dispersion, such as particles in a colloid, droplets in a cloud, crystals in a rock, or polymer macromolecules in a solution or a solid polymer mass. Polymers can be described by molecular mass distribution; a population of particles can be described by size, surface area, and/or mass distribution; and thin films can be described by film thickness distribution. IUPAC has deprecated the use of the term ''polydispersity index'', having replaced it with the term ''dispersity'', represented by the symbol Đ (pronounced D-strokeStepto, R. F. T.; Gilbert, R. G.; Hess, M.; Jenkins, A. D.; Jones, R. G.; Kratochvíl P. (2009).Dispersity in Po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilkinson
Wilkinson or Wilkinsons may refer to: Businesses and brands * Wilko, formerly Wilkinson Hardware, a British retail chain * Wilkinson Sword, a British manufacturer of razor blades, formerly swords, motorbikes and other products ** Wilkinson TMC, their touring motorcycle model built 1911 to 1916 * Wilkinson plc, a British firm of chandelier manufacturers and repairers People * Wilkinson (surname), including a list of people * Wilkinson (musician), an English musician * Wilkinson baronets, a British hereditary title * The Wilkinsons, a Canadian country music trio ** ''The Wilkinsons'' (TV series) Places * Wilkinson County, Georgia, U.S. * Wilkinson, Illinois, U.S. * Wilkinson, Indiana, U.S. * Wilkinson, Minnesota, U.S. ** Wilkinson Township, Cass County, Minnesota, the wider area * Wilkinson, Mississippi, U.S. ** Wilkinson County, Mississippi, the wider county * Wilkinson, West Virginia, U.S. * Wilkinson, Wisconsin, U.S. * Wilkinson Lake, in Minnesota, U.S. * Wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
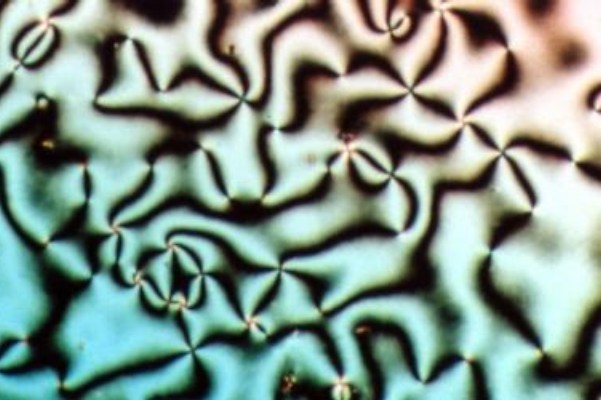
Nematic
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |