|
Polypyrrole
Polypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields. History Some of the first examples of PPy were reported in 1919 by Angeli and Pieroni, who reported the formation of pyrrole blacks from pyrrole magnesium bromide. Since then pyrrole oxidation reaction has been studied and reported in scientific literature. Work on conductive polymers including polypyrrole, polythiophene, polyaniline, and polyacetylene was awarded the Nobel Prize in Chemistry in 2000 to Alan J. Heeger, Alan G. MacDiarmid and Hideki Shirakawa . Synthesis Different methods can be used to synthesize PPy, but the most common are electrochemical synthesis and chemical oxidation. Chemical oxidation of pyrrole: :n C4H4NH + 2n FeCl3 → (C4H2NH)n + 2n FeCl2 + 2n HCl The process is thought to occur via the formation of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, . Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties, structure, bonding Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrroles
Pyrrole is a heterocyclic, Aromaticity, aromatic, organic compound, a five-membered Ring (chemistry), ring with the chemical formula, formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, . Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties, structure, bonding Pyrrole is a colorless volatility (chemistry), volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
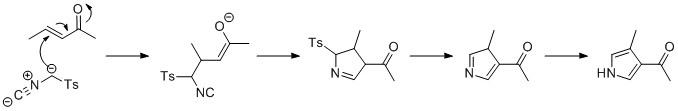
Pyrrole Electro-polymerisation
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, . Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties, structure, bonding Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conducting Polymer
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The main advantage of conductive polymers is that they are easy to process, mainly by dispersion. Conductive polymers are generally not thermoplastics, ''i.e.'', they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques. History Polyaniline was first described in the mid-19th century by Henry Letheby, who investigated the electrochemical and chemical oxidation products of aniline in acidic media. He noted that the reduced form was colourless but the oxidized forms were deep blue. The first highly-conduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Cation
Radical cations are denoted M^. Salts of these species have been isolated in the cases of dibenzocyclooctatetraene, various tertiary amines, and some polymethylated derivatives of azulene. Radical cations, like radical anions, have one unpaired electron, i.e. they are paramagnetic. Mass spectrometry Radical cations appear prominently in mass spectrometry. When a gas-phase molecule is subjected to electron ionization one electron is abstracted by an electron in the electron beam to create a radical cation M+.. This species represents the molecular ion or parent ion. A typical mass spectrum shows multiple signals because the molecular ion fragments into a complex mixture of ions and uncharged radical species. For example, the methanol radical cation fragments into a methenium cation and a hydroxyl radical. In naphthalene the unfragmented radical cation is by far the most prominent peak in the mass spectrum. Secondary species are generated from proton gain (M+1) and proton loss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst Support
In chemistry, a catalyst support or carrier is a material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts is mainly promoted by atoms present at the accessible surface of the material. Consequently, great effort is made to maximize the specific surface area of a catalyst. One popular method for increasing surface area involves distributing the catalyst over the surface of the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of activated carbon, alumina, and silica. Applying catalysts to supports Two main methods are used to prepare supported catalysts. In the impregnation method, a suspension of the solid support is treated with a solution of a precatalyst, and the resulting material is then activated under conditions that will convert the precatalyst (often a metal salt) to a more active state, perhaps the metal itself. In such cases, the cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Semiconductors
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are injected from appropriate electrodes or are introduced by doping or photoexcitation. General properties In molecular crystals the energetic separation between the top of the valence band and the bottom conduction band, i.e. the band gap, is typically 2.5–4 eV, while in inorganic semiconductors the band gaps are typically 1–2 eV. This implies that molecular crystals are, in fact, insulators rather than semiconductors in the conventional sense. They become semiconducting only when charge carriers are either injected from the electrodes or generated by intentional or unintentional doping. Charge carriers can also be g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Electronics
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. It provides a potential means to extend Moore's Law beyond the foreseen limits of small-scale conventional silicon integrated circuits. Molecular scale electronics Molecular scale electronics, also called single-molecule electronics, is a branch of nanotechnology that uses single molecules, or nanoscale collections of single molecules, as electronic components. Because single molecules constitute the smallest stable structures possible, this miniaturization is the ultimate goal for shrinking electrical circuits. Conventional electronic devices are traditionally made from bulk materials. Bulk methods have inherent limits, and are growing increasingly demanding and costly. Thus, the idea was born that the components could instead be built up atom by atom in a chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. Polymers are studied in the fields of polymer science (which includes polymer chemistry and polymer physics), biophy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( or units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life. Some tetrapyrroles form the active core of compounds with crucial biochemical roles in living systems, such as hemoglobin and chlorophyll. In these two molecules, in particular, the pyrrole macrocycle ring frames a metal atom, that forms a coordination compound with the pyrroles and plays a central role in the biochemical function of those molecules. Structure Linear tetrapyrroles (called bilanes) include: *Heme breakdown products (e.g., bilirubin, biliverdin) * Phycobilins (found in cyanobacteria) * Luciferins as found in dinoflagellates and euphausiid shrimps (krill) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Semiconductor
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are injected from appropriate electrodes or are introduced by doping or photoexcitation. General properties In molecular crystals the energetic separation between the top of the valence band and the bottom conduction band, i.e. the band gap, is typically 2.5–4 eV, while in inorganic semiconductors the band gaps are typically 1–2 eV. This implies that molecular crystals are, in fact, insulators rather than semiconductors in the conventional sense. They become semiconducting only when charge carriers are either injected from the electrodes or generated by intentional or unintentional doping. Charge carriers can also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |