|
Polyampholytes
Polyampholytes are polymers that contain both positively charged (cationic) and negatively charged (anionic) functional groups within the same molecule. Their unique structure allows them to exhibit amphoteric behavior, meaning they can interact with a range of substances depending on the surrounding pH, making them useful in applications like drug delivery, water treatment, and biomaterials. Polyampholytes can exist as either linear water-soluble polyelectrolytes or as cross-linked structures. Weakly cross-linked polyampholytes swell in water, forming hydrogels. The swelling properties of these hydrogels are highly dependent on the solution pH and its relation to the polyampholyte’s isoelectric point. The isoelectric point of polyampholytes is the pH at which the polymer exhibits no net charge, balancing its positive and negative charges. This point is important because it dictates the net charge of polyampholyte macromolecules at different pH levels. At a pH less than the isoe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes ( salts) and polymers (high molecular weight compounds) and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describe their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogel
A hydrogel is a Phase (matter), biphasic material, a mixture of Porosity, porous and Permeation, permeable solids and at least 10% of water or other interstitial fluid. The solid phase is a water Solubility, insoluble three dimensional network of polymers, having absorbed a large amount of water or biological fluids. Hydrogels have several applications, especially in the biomedical area, such as in hydrogel dressing. Many hydrogels are synthetic, but some are derived from natural materials. The term "hydrogel" was coined in 1894. Chemistry Classification The crosslinks which bond the polymers of a hydrogel fall under two general categories: physical hydrogels and chemical hydrogels. Chemical hydrogels have Covalent bond, covalent cross-linking bonds, whereas physical hydrogels have non-covalent bonds. Chemical hydrogels can result in strong reversible or irreversible gels due to the covalent bonding. Chemical hydrogels that contain reversible covalent cross-linking bonds, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoelectric Point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electric charge, electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also used. For concision, brevity, this article uses pI. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons#In Physics and biochemistry, protons (H+). Surfaces naturally charge to form a double layer (interfacial), double layer. In the common case when the surface charge-determining ions are H+/HO−, the net surface charge is affected by the pH of the liquid in which the solid is submerged. The pI value can affect the solubility of a molecule at a given pH. Such molecules have minimum solubility in water or salt solutions at the pH that corresponds to their pI and often precipitate out of Solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelatin
Gelatin or gelatine () is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics. Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, dips, and yogurts. Gelatin for cooking comes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of biomineralization, mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the Gut (anatomy), gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomaterial
A biomaterial is a substance that has been Biological engineering, engineered to interact with biological systems for a medical purpose – either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a Medical diagnosis, diagnostic one. The corresponding field of study, called biomaterials science or biomaterials engineering, is about fifty years old. It has experienced steady growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. A biomaterial is different from a biological material, such as bone, that is produced by a biological system. However, "biomaterial" and "biological material" are often used interchangeably. Further, the word "bioterial" has been proposed as a potential alternate word for biologically-produced materials such as bone, or fungal biocomposites. Additi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
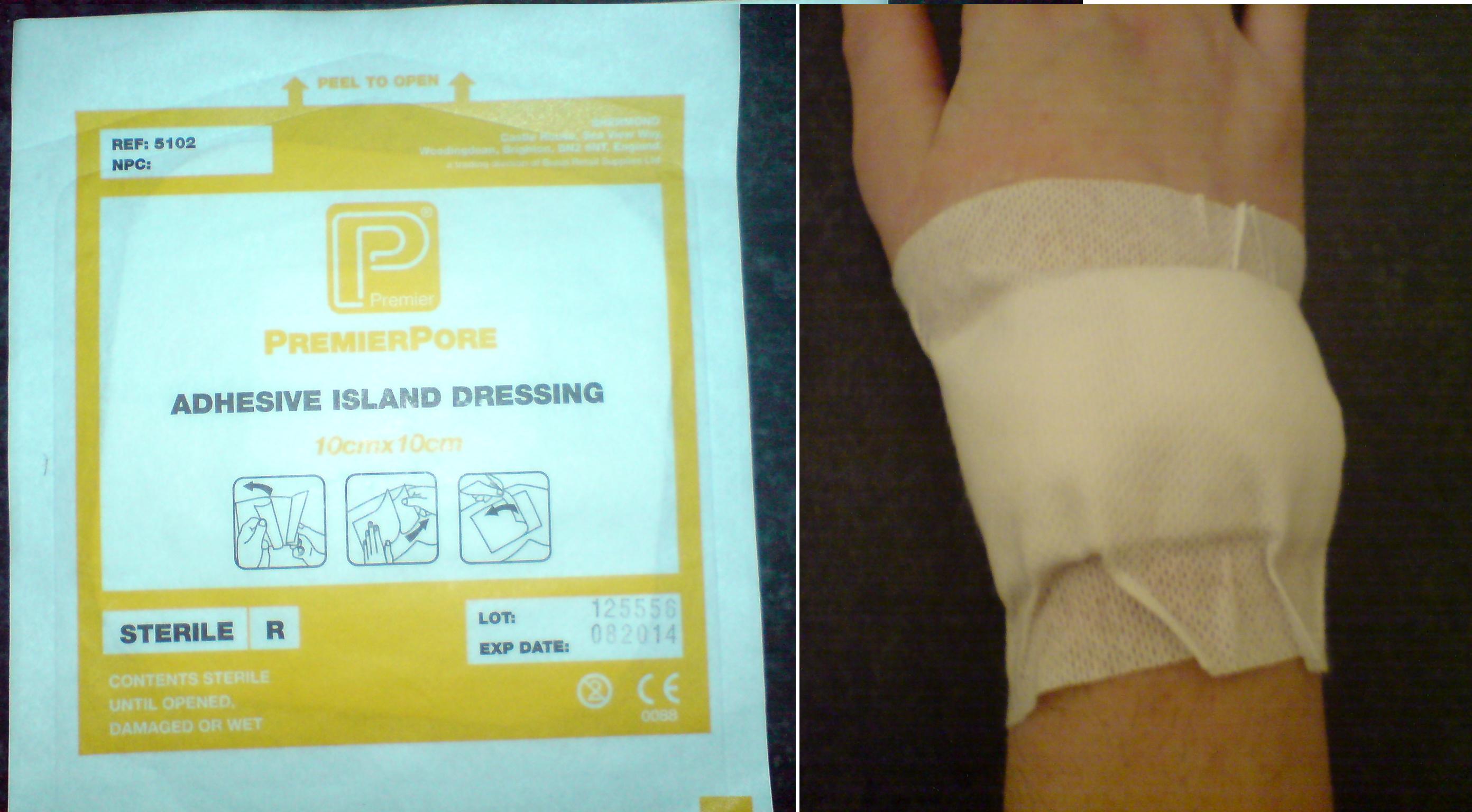
Wound Dressings
A dressing or compress is a piece of material such as a pad applied to a wound to promote healing and protect the wound from further harm. A dressing is designed to be in direct contact with the wound, as distinguished from a bandage, which is most often used to hold a dressing in place. Modern dressings are sterile. Medical uses A dressing can have a number of purposes, depending on the type, severity and position of the wound, although all purposes are focused on promoting recovery and protecting from further harm. Key purposes of a dressing are: * Stop bleeding – to help to seal the wound to expedite the clotting process; * Protection from infection – to defend the wound against germs and mechanical damage; * Absorb exudate – to soak up blood, plasma, and other fluids exuded from the wound, containing it/them in one place and preventing maceration; * Ease pain – either by a medicated analgesic effect, compression or simply preventing pain from further trauma; * Deb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryoprotectant
A cryoprotectant is a substance used to protect biological tissue from freezing damage (i.e. that due to ice formation). Arctic and Antarctic insects, fish and amphibians create cryoprotectants ( antifreeze compounds and antifreeze proteins) in their bodies to minimize freezing damage during cold winter periods. Cryoprotectants are also used to preserve living materials in the study of biology and to preserve food products. For years, glycerol has been used in cryobiology as a cryoprotectant for blood cells and bull sperm, allowing storage in liquid nitrogen at temperatures around −196 °C. However, glycerol cannot be used to protect whole organs from damage. Instead, many biotechnology companies are researching the development of other cryoprotectants more suitable for such uses. A successful discovery may eventually make possible the bulk cryogenic storage (or "banking") of transplantable human and xenobiotic organs. A substantial step in that direction has already occu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |