|
Photoinhibition
Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga, or cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light. History The first measurements of photoinhibition were published in 1956 by Bessel Kok. Even in the very first studies, it was obvious that plants have a repair mechanism that continuously repairs photoinhibitory damage. In 1966, Jones and Kok measured the action spectrum of photoinhibition and found that ultraviolet light is highly photoinhibitory. The visible-l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoinitiator
A photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesives and dental restoratives). Some small molecules in the atmosphere can also act as photoinitiators by decomposing to give free radicals (in photochemical smog). For instance, nitrogen dioxide (NO2) is produced in large quantities by gasoline-burning internal combustion engines. NO2 in the troposphere gives smog its brown coloration and catalyzes production of toxic ground-level ozone (O3). Molecular oxygen (O2) also serves as a photoinitiator in the stratosphere, breaking down into atomic oxygen and combining with O2 in order to form the ozone in the ozone layer. Reactions Photoinitators can create reactive species by different pathways including photodissociation and electron transfer. As an example of dissociation, hydrogen peroxide ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloramphenicol
Chloramphenicol is an antibiotic An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, ... useful for the treatment of a number of bacterial infections. This includes use as an eye ointment to treat conjunctivitis. By mouth or by intravenous, injection into a vein, it is used to treat meningitis, plague (disease), plague, cholera, and typhoid fever. Its use by mouth or by injection is only recommended when safer antibiotics cannot be used. Monitoring both blood levels of the medication and blood cell levels every two days is recommended during treatment. Common side effects include bone marrow suppression, nausea, and diarrhea. The bone marrow suppression may result in death. To reduce the risk of side effects treatment duration should be as short as possible. People with liver or kidne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Fixation
Biological carbon fixation or сarbon assimilation is the process by which inorganic carbon (particularly in the form of carbon dioxide) is converted to organic compounds by living organisms. The compounds are then used to store energy and as structure for other biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use a process called chemosynthesis in the absence of sunlight. Organisms that grow by fixing carbon are called autotrophs, which include photoautotrophs (which use sunlight), and lithoautotrophs (which use inorganic oxidation). Heterotrophs are not themselves capable of carbon fixation but are able to grow by consuming the carbon fixed by autotrophs or other heterotrophs. "Fixed carbon", "reduced carbon", and "organic carbon" may all be used interchangeably to refer to various organic compounds. Chemosynthesis is carbon fixation driven by chemical energy, rather than from sunlight. Sulfur- and hydrogen-oxidizing bacteria often use the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
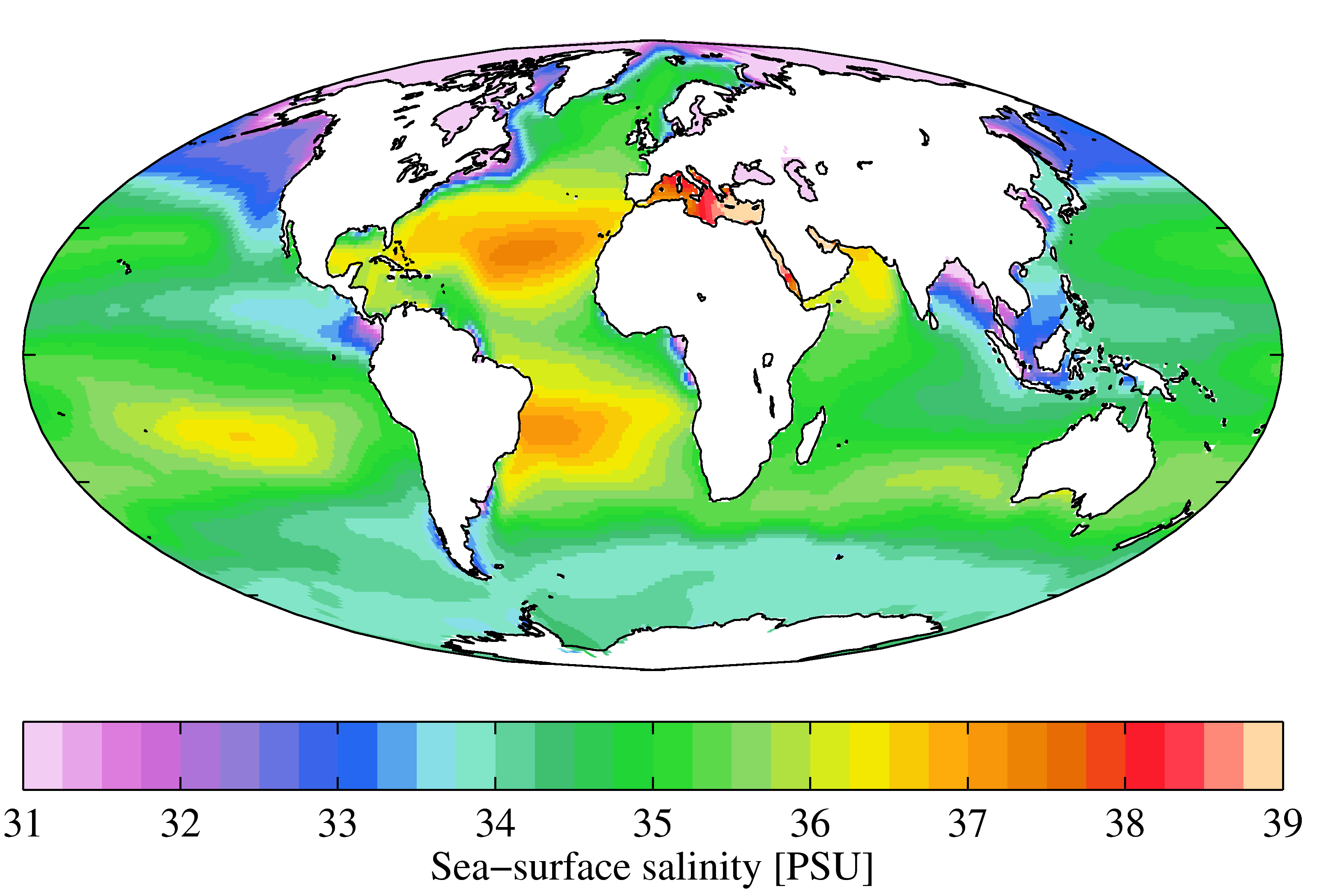
Salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsicum Annuum
''Capsicum annuum'' is a species of the plant genus '' Capsicum'' native to southern North America, the Caribbean, and northern South America. This species is the most common and extensively cultivated of the five domesticated capsicums. The species encompasses a wide variety of shapes and sizes of peppers, including sweet bell peppers and some chili pepper varieties such as jalapeños, New Mexico chile, and cayenne peppers. Cultivars descended from the wild American bird pepper are still found in warmer regions of the Americas. In the past, some woody forms of this species have been called '' C. frutescens'', but the features that were used to distinguish those forms appear in many populations of ''C. annuum'' and are not consistently recognizable features in ''C. frutescens'' species. Characteristics Although the species name ''annuum'' means 'annual' (from the Latin ''annus'' "year"), the plant is not an annual but is frost tender. In the absence of winter frosts it ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lincomycin
Lincomycin is a lincosamide antibiotic that comes from the actinomycete ''Streptomyces lincolnensis''. A related compound, clindamycin, is derived from lincomycin by using thionyl chloride to replace the 7-hydroxy group with a chlorine atom with inversion of chirality. It was released for medical use in September 1964. Uses Although similar in antibacterial spectrum and mechanism of action to macrolides, lincomycin is also effective against other organisms including actinomycetes and some species of ''Mycoplasma'' and '' Plasmodium''. However, because of its adverse effects and toxicity, it is rarely used today and reserved for patients allergic to penicillin or where bacteria have developed resistance. Clinical pharmacology Intramuscular administration of a single dose of 600 mg of Lincomycin produces average peak serum levels of 11.6 µg/mL at 60 min, and maintains therapeutic levels for 17 h to 20 h, for most susceptible gram-positive organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Equation
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter ( molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron–sulfur Protein
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d. Cytochrome function is linked to the reversible redox change from ferrous (Fe(II)) to the ferric (Fe(III)) oxidation state of the iron found in the heme core. In addition to the classification by the IUBMB into four cytochrome classes, several additional classifications such as cytochrome o and cytochrome P450 can be found in biochemical literature. History Cytochromes were initially described in 1884 by Charles Alexander MacMunn as respiratory pigments (myohematin or histohematin). In the 1920s, Keilin rediscovered these respiratory pigments and named them th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
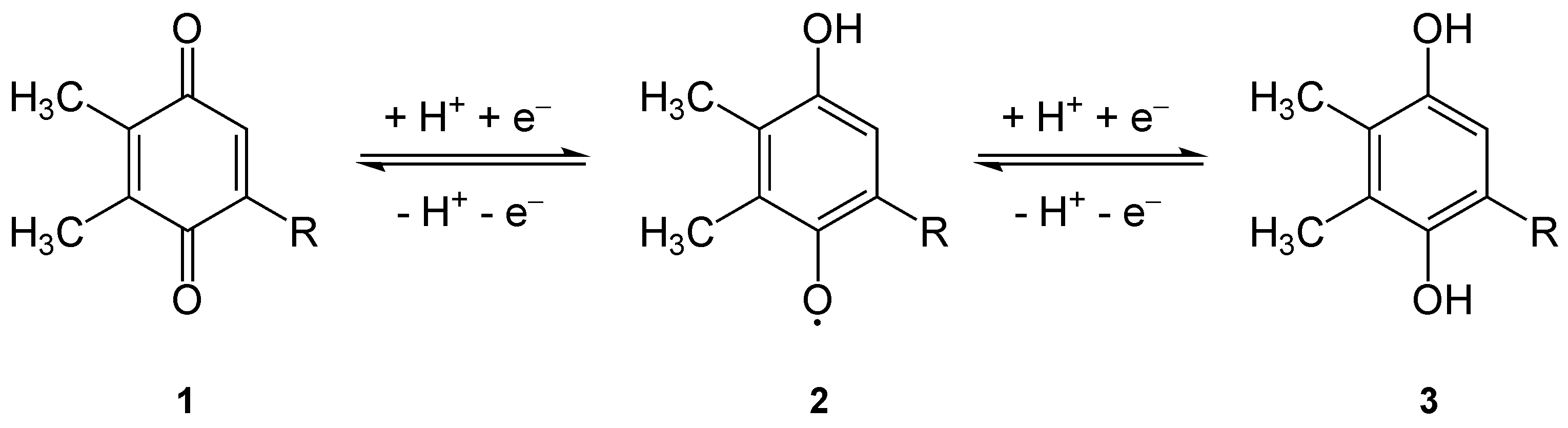
Plastoquinone
Plastoquinone (PQ) is an isoprenoid quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4- benzoquinone molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 (which has 3 isoprenyl side units instead of 9) as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar, anchoring the molecule within the inner section of a lipid bilayer, where the hydrophobic tails are usually found. Plastoquinones are very structurally similar to ubiquinone, or coenzyme Q10, differing by the length of the isoprenyl side chain, replacement of the methoxy groups with methyl groups, and removal of the methyl group in the 2 position on the quinone. Like ubiquinone, it can come in several oxidation states ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |