|
Lincomycin
Lincomycin is a lincosamide antibiotic that comes from the actinomycete ''Streptomyces lincolnensis''. A related compound, clindamycin, is derived from lincomycin by using thionyl chloride to replace the 7-hydroxy group with a chlorine atom with inversion of chirality. It was released for medical use in September 1964. Uses Although similar in antibacterial spectrum and mechanism of action to macrolides, lincomycin is also effective against other organisms including actinomycetes and some species of ''Mycoplasma'' and '' Plasmodium''. However, because of its adverse effects and toxicity, it is rarely used today and reserved for patients allergic to penicillin or where bacteria have developed resistance. Clinical pharmacology Intramuscular administration of a single dose of 600 mg of Lincomycin produces average peak serum levels of 11.6 µg/mL at 60 min, and maintains therapeutic levels for 17 h to 20 h, for most susceptible gram-positive organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lincosamide
Lincosamides are a class of antibiotics, which include lincomycin, clindamycin, and pirlimycin. Structure Lincosamides consist of a pyrrolidine ring linked to a pyranose moiety (methylthio-lincosamide) via an amide bond. Hydrolysis of lincosamides, specifically lincomycin, splits the molecule into its building blocks of the sugar and proline moieties. Both of these derivatives can conversely be recombined into the drug itself or a derivative. Synthesis Biosynthesis of lincosamides occurs through a biphasic pathway, in which propylproline and methylthiolincosamide are independently synthesized immediately before condensation of the two precursor molecules. Condensation of the propylproline carboxyl group with the methylthiolincosamide amine group via an amide bond forms ''N''-demethyllincomycin. ''N''-Demethyllincomycin is subsequently methylated via ''S''-adenosyl methionine to produce lincomycin A. Lincomycin is naturally produced by bacteria species, namely ''Streptomyces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lincosamides
Lincosamides are a class of antibiotics, which include lincomycin, clindamycin, and pirlimycin. Structure Lincosamides consist of a pyrrolidine ring linked to a pyranose moiety (methylthio-lincosamide) via an amide bond. Hydrolysis of lincosamides, specifically lincomycin, splits the molecule into its building blocks of the sugar and proline moieties. Both of these derivatives can conversely be recombined into the drug itself or a derivative. Synthesis Biosynthesis of lincosamides occurs through a biphasic pathway, in which propylproline and methylthiolincosamide are independently synthesized immediately before condensation of the two precursor molecules. Condensation of the propylproline carboxyl group with the methylthiolincosamide amine group via an amide bond forms ''N''-demethyllincomycin. ''N''-Demethyllincomycin is subsequently methylated via ''S''-adenosyl methionine to produce lincomycin A. Lincomycin is naturally produced by bacteria species, namely ''Streptomyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clindamycin
Clindamycin is an antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media (middle ear infections), and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant ''Staphylococcus aureus'' (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina. Common side effects include nausea and vomiting, diarrhea, rashes, and pain at the site of injection. It increases the risk of hospital-acquired ''Clostridium difficile'' colitis about fourfold and thus is only recommended when other antibiotics are not appropriate. Alternative antibiotics may be recommended as a result. It appears to be generally safe in pregnancy. It is of the lincosamide class and works by blocking bact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinomycete
The Actinomycetales is an order of Actinomycetota. A member of the order is often called an actinomycete. Actinomycetales are generally gram-positive and anaerobic and have mycelia in a filamentous and branching growth pattern. Some actinomycetes can form rod- or coccoid-shaped forms, while others can form spores on aerial hyphae. Actinomycetales bacteria can be infected by bacteriophages, which are called actinophages. Actinomycetales can range from harmless bacteria to pathogens with resistance to antibiotics. Reproduction Actinomycetales have 2 main forms of reproduction: spore formation and hyphae fragmentation. During reproduction, Actinomycetales can form conidiophores, sporangiospores, and oidiospores. In reproducing through hyphae fragmentation, the hyphae formed by Actinomycetales can be a fifth to half the size of fungal hyphae, and bear long spore chains. Presence and associations Actinomycetales can be found mostly in soil and decaying organic matter, as well as in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
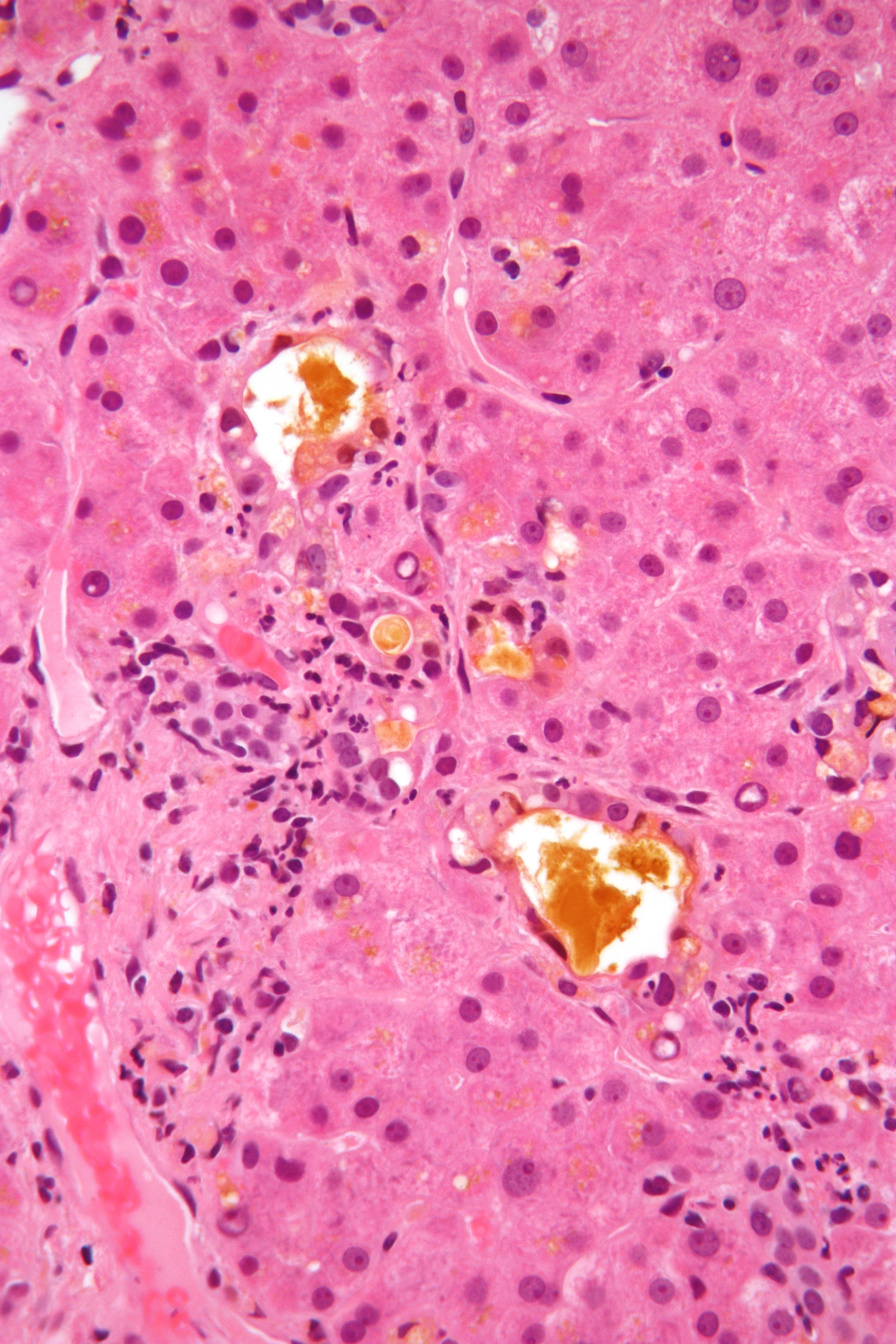
Bile
Bile (from Latin ''bilis''), or gall, is a dark-green-to-yellowish-brown fluid produced by the liver of most vertebrates that aids the digestion of lipids in the small intestine. In humans, bile is produced continuously by the liver (liver bile) and stored and concentrated in the gallbladder. After eating, this stored bile is discharged into the duodenum. The composition of hepatic bile is (97–98)% water, 0.7% bile salts, 0.2% bilirubin, 0.51% fats (cholesterol, fatty acids, and lecithin), and 200 meq/L inorganic salts. The two main pigments of bile are bilirubin, which is yellow, and its oxidised form biliverdin, which is green. When mixed, they are responsible for the brown color of feces. About 400 to 800 millilitres of bile is produced per day in adult human beings. Function Bile or gall acts to some extent as a surfactant, helping to emulsify the lipids in food. Bile salt anions are hydrophilic on one side and hydrophobic on the other side; consequently, they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
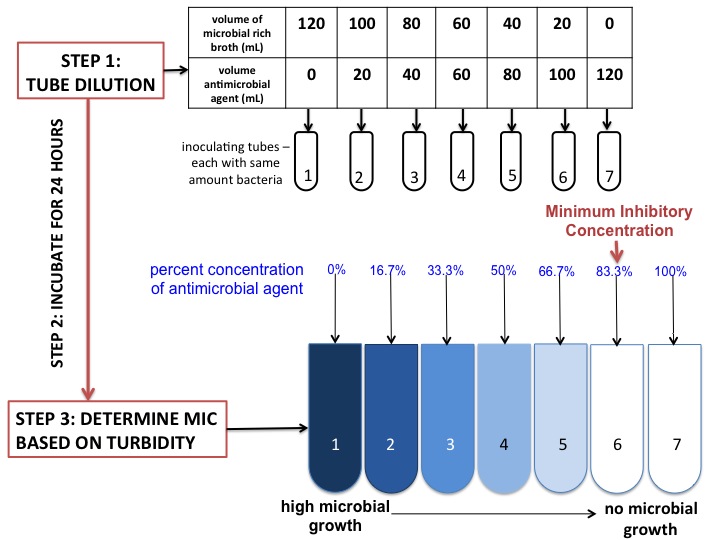
Minimum Inhibitory Concentration
In microbiology, the minimum inhibitory concentration (MIC) is the lowest concentration of a chemical, usually a drug, which prevents visible growth of a bacterium or bacteria. MIC depends on the microorganism, the affected human being (in vivo only), and the antibiotic itself. It is often expressed in micrograms per milliliter (μg/mL) or milligrams per liter (mg/L). The MIC is determined by preparing solutions of the chemical in vitro at increasing concentrations, incubating the solutions with separate batches of cultured bacteria, and measuring the results using agar dilution or broth microdilution. Results have been graded into susceptible (often called sensitive), increased exposure, or resistant to a particular antimicrobial by using a breakpoint. Breakpoints are agreed upon values, published in guidelines of a reference body, such as the U.S. Clinical and Laboratory Standards Institute (CLSI), the British Society for Antimicrobial Chemotherapy (BSAC) or the European Commit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ergothioneine
Ergothioneine is a naturally occurring amino acid and is a thiourea derivative of histidine, containing a sulfur atom on the imidazole ring. This compound occurs in relatively few organisms, notably actinomycetota, cyanobacteria, and certain fungi. Ergothioneine was discovered in 1909 and named after the ergot fungus from which it was first purified, with its structure being determined in 1911. In humans, ergothioneine is acquired exclusively through the diet and accumulates in erythrocytes, bone marrow, liver, kidney, seminal fluid, and eyes. Although the effect of ergothioneine ''in vivo'' is under preliminary research, its physiological role in humans is unknown. Ergothioneine is sold as a dietary supplement. Metabolism and sources Ergothioneine has been found in bacteria, plants, and animals, sometimes at millimolar levels. Foods found to contain ergothioneine include liver, kidney, black beans, kidney bean, and oat bran, with the highest levels in bolete and oyster mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transaldolase
Transaldolase is an enzyme () of the non-oxidative phase of the pentose phosphate pathway. In humans, transaldolase is encoded by the ''TALDO1'' gene. The following chemical reaction is catalyzed by transaldolase: : sedoheptulose 7-phosphate + glyceraldehyde 3-phosphate \rightleftharpoons erythrose 4-phosphate + fructose 6-phosphate Clinical significance The pentose phosphate pathway has two metabolic functions: (1) generation of nicotinamide adenine dinucleotide phosphate (reduced NADPH), for reductive biosynthesis, and (2) formation of ribose, which is an essential component of ATP, DNA, and RNA. Transaldolase links the pentose phosphate pathway to glycolysis. In patients with deficiency of transaldolase, there's an accumulation of erythritol (from erythrose 4-phosphate), D-arabitol, and ribitol. The deletion in 3 base pairs in the ''TALDO1'' gene results in the absence of serine at position 171 of the transaldolase protein, which is part of a highly conserved region, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the targ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycothiol
Mycothiol (MSH or AcCys-GlcN-Ins) is an unusual thiol compound found in the Actinomycetota. It is composed of a cysteine residue with an acetylated amino group linked to glucosamine, which is then linked to inositol. The oxidized, disulfide form of mycothiol (MSSM) is called mycothione, and is reduced to mycothiol by the flavoprotein mycothione reductase. Mycothiol biosynthesis and mycothiol-dependent enzymes such as mycothiol-dependent formaldehyde dehydrogenase and mycothione reductase have been proposed to be good drug targets for the development of treatments for tuberculosis. See also * Glutathione Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ..., analogous function in other Bacteria * Bacillithiol References {{reflist Mycobacterium tuberculosis is extraordinarily se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |