|
Phosphorylase
In biochemistry, phosphorylases are enzymes that catalyze the addition of a phosphate group from an inorganic phosphate (phosphate+hydrogen) to an acceptor. :A-B + P A + P-B They include allosteric enzymes that catalyze the production of glucose-1-phosphate from a glucan such as glycogen, starch or maltodextrin. Phosphorylase is also a common name used for glycogen phosphorylase in honor of Earl W. Sutherland Jr., who in the late 1930s discovered it as the first phosphorylase. Function Phosphorylases should not be confused with phosphatases, which remove phosphate groups. In more general terms, phosphorylases are enzymes that catalyze the addition of a phosphate group from an inorganic phosphate (phosphate + hydrogen) to an acceptor, not to be confused with a phosphatase (a hydrolase) or a kinase (a phosphotransferase). A phosphatase removes a phosphate group from a donor using water, whereas a kinase transfers a phosphate group from a donor (usually ATP) to an accep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
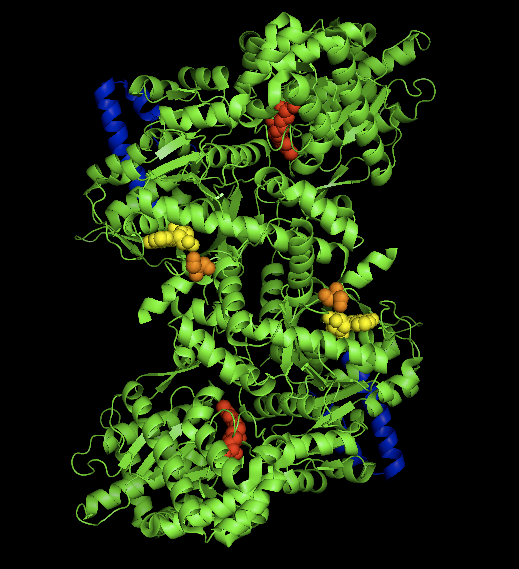
Glycogen Phosphorylase
Glycogen phosphorylase is one of the phosphorylase enzymes (). Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects. Mechanism Glycogen phosphorylase breaks up glycogen into glucose subunits (see also figure below): (α-1,4 glycogen chain)n + Pi ⇌ (α-1,4 glycogen chain)n-1 + α-D-glucose-1-phosphate. Glycogen is left with one fewer glucose molecule, and the free glucose molecule is in the form of glucose-1-phosphate. In order to be used for metabolism, it must be converted to glucose-6-phosphate by the enzyme phosphoglucomutase. Although the reaction is reversible in vitro, within the cell the enzyme only works in the forward direction as shown below because the concentration of inorganic phosphate is much higher than that of gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen Storage Disease Type V
Glycogen storage disease type V (GSD5, GSD-V), also known as McArdle's disease, is a metabolic disorder, one of the metabolic myopathies, more specifically a muscle glycogen storage disease, caused by a deficiency of myophosphorylase. Its incidence is reported as one in 100,000, roughly the same as glycogen storage disease type I. The disease was first reported in 1951 by British physician Brian McArdle of Guy's Hospital, London. Signs and symptoms Onset of symptoms and diagnostic delay In the classic phenotype, the onset of this disease is usually noticed in childhood, but often not diagnosed until the third or fourth decade of life, frequently due to misdiagnosis and dismissal of symptoms. The median age of symptom onset is 3 years, with the median diagnostic delay being 29 years. Misdiagnosis is overwhelmingly common, with approximately 90% of patients being misdiagnosed, and approximately 62% receiving multiple misdiagnoses before a correct diagnosis. The prolonged diagno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Nucleoside Phosphorylase
Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase () is an enzyme that in humans is encoded by the ''NP'' gene. It catalyzes the chemical reaction :purine nucleoside + phosphate \rightleftharpoons purine + alpha-D-ribose 1-phosphate Thus, the two substrates of this enzyme are a purine nucleoside and phosphate, whereas its products are a purine and alpha-D-ribose 1-phosphate. Nomenclature This enzyme belongs to the family of glycosyltransferases, specifically the pentosyltransferases. The systematic name of this enzyme class is purine-nucleoside:phosphate ribosyltransferase. Other names in common use include: * inosine phosphorylase * PNPase * PUNPI * PUNPII * inosine-guanosine phosphorylase * nucleotide phosphatase * purine deoxynucleoside phosphorylase * purine deoxyribonucleoside phosphorylase * purine nucleoside phosphorylase * purine ribonucleoside phosphorylas This enzyme participates in 3 metabolic pathways: purine metabolism, pyrimidine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltodextrin Phosphorylase
Maltodextrin phosphorylase is a phosphorylase enzyme ( EC 2.4.1.1), more specifically one type of glycosyltransferase ( EC 2.4). Maltodextrin phosphorylase plays a critical role in maltodextrin metabolism in E. coli. This bacterial enzyme, often referred to as MalP, catalyzes the phosphorolysis of an α-1,4-glycosidic bond in maltodextrins, removing the non-reducing glucosyl residues of linear oligosaccharides as glucose-1-phosphate (Glc1P). Phosphorylases are well-regarded for their allosteric effects on metabolism, however MalP exhibits no allosteric properties. It has a higher affinity for linear oligosaccharides than the related glycogen phosphorylase. Mechanism Maltodextrin phosphorylase facilitates maltodextrin metabolism through phosphorolysis of nonreducing glucosyl residues in order to produce Glc1P. MalP has a higher affinity for short, linear α-1,4 linked glucose oligosaccharides, and consequently appears to act on maltodextrin degradation products. Without MalP, la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earl Wilbur Sutherland Jr
Earl Wilbur Sutherland Jr. (November 19, 1915 – March 9, 1974) was an American pharmacologist and biochemist born in Burlingame, Kansas. Sutherland won a Nobel Prize in Physiology or Medicine in 1971 "for his discoveries concerning the mechanisms of the action of hormones", especially epinephrine, via second messengers, namely cyclic adenosine monophosphate, or cyclic AMP. Early life and education Sutherland was born on November 19, 1915, in Burlingame, Kansas. The second youngest of six children, he was raised by his mother, Edith M. Hartshorn, and his father, Earl W. Sutherland.Gale Group (2006)"World of Scientific Discovery on Earl Sutherland". World of Scientific Discovery. Though his father, who was originally from Wisconsin, had attended Grinnell College for two years, he ultimately led an agrarian lifestyle that took him to both New Mexico and Oklahoma before settling down in Burlingame to raise a family. Edith, a Missouri native, had some training in nursing at what wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch Phosphorylase
Starch phosphorylase is a form of phosphorylase similar to glycogen phosphorylase, except that it acts upon starch instead of glycogen Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. It is the main storage form of glucose in the human body. Glycogen functions as one of three regularly used forms .... The plant alpha-glucan phosphorylase, commonly called starch phosphorylase (EC 2.4.1.1), is largely known for the phosphorolytic degradation of starch. Starch phosphorylase catalyzes the reversible transfer of glucosyl units from glucose-1-phosphate to the nonreducing end of alpha-1,4-D-glucan chains with the release of phosphate. Two distinct forms of starch phosphorylase, plastidic phosphorylase and cytosolic phosphorylase, have been consistently observed in higher plants. External links * * http://www.genome.ad.jp/dbget-bin/www_bget?ko+K00688 ** Rathore RS, Garg N, Garg S, Kumar A. Crit Rev Biot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose-1-phosphate
Glucose 1-phosphate (also called Cori ester) is a glucose molecule with a phosphate group on the 1'-carbon. It can exist in either the α- or β-anomeric form. Reactions of α-glucose 1-phosphate Catabolic In glycogenolysis, it is the direct product of the reaction in which glycogen phosphorylase cleaves off a molecule of glucose from a greater glycogen structure. A deficiency of muscle glycogen phosphorylase is known as glycogen storage disease type V (McArdle Disease). To be utilized in cellular catabolism it must first be converted to glucose 6-phosphate by the enzyme phosphoglucomutase in a free equilibrium. One reason that cells form glucose 1-phosphate instead of glucose during glycogen breakdown is that the very polar phosphorylated glucose cannot leave the cell membrane and so is marked for intracellular catabolism. Phosphoglucomutase-1 deficiency is known as glycogen storage disease type 14 (GSD XIV). Anabolic In glycogenesis, free glucose 1-phosphate can also react w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen Storage Disease Type VI
Glycogen storage disease type VI (GSD VI) is a type of glycogen storage disease caused by a deficiency in liver glycogen phosphorylase or other components of the associated phosphorylase cascade system. It is also known as "Hers' disease", after Henri G. Hers, who characterized it in 1959. The scope of GSD VI now also includes glycogen storage disease type VIII, IX (caused by phosphorylase b kinase deficiency) and X (deficiency protein kinase A). The incidence of GSD VI is approximately 1 case per 65,000–85,000 births, representing approximately 30% all cases of glycogen storage disease. Signs and symptoms Patients generally have a benign course, and typically present with hepatomegaly and growth retardation early in childhood. Mild hypoglycemia, hyperlipidemia, and hyperketosis may occur. Lactic acid and uric acid Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the Chemical formula, formula C5H4N4O3. It forms ions and salts known a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polynucleotide Phosphorylase
Polynucleotide Phosphorylase (PNPase) is a bifunctional enzyme with a phosphorolytic 3' to 5' exoribonuclease activity and a 3'-terminal oligonucleotide polymerase activity. That is, it dismantles the RNA chain starting at the 3' end and working toward the 5' end. It also synthesizes long, highly heteropolymeric tails ''in vivo''. It accounts for all of the observed residual polyadenylation in strains of ''Escherichia coli'' missing the normal polyadenylation enzyme. Discovered by Marianne Grunberg-Manago working in Severo Ochoa's lab in 1955, the RNA-polymerization activity of PNPase was initially believed to be responsible for DNA-dependent synthesis of messenger RNA, a notion that was disproven by the late 1950s. It is involved in mRNA processing and degradation in bacteria, plants, and animals. In humans, the enzyme is encoded by the gene. In its active form, the protein forms a ring structure consisting of three PNPase molecules. Each PNPase molecule consists of two R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. It is the main storage form of glucose in the human body. Glycogen functions as one of three regularly used forms of energy reserves, creatine phosphate being for very short-term, glycogen being for short-term and the triglyceride stores in adipose tissue (i.e., body fat) being for long-term storage. Protein, broken down into amino acids, is seldom used as a main energy source except during starvation and glycolytic crisis ''(see bioenergetic systems)''. In humans, glycogen is made and stored primarily in the cells of the liver and skeletal muscle. In the liver, glycogen can make up 5–6% of the organ's fresh weight: the liver of an adult, weighing 1.5 kg, can store roughly 100–120 grams of glycogen. In skeletal muscle, glycogen is found in a low concentration (1–2% of the muscle mass): the skeletal muscle of an adult weighing 70 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. As a result, kinase produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate groups to an acceptor, nor with phosphatases, which remove phosphate groups (dephosphorylation). The phosphorylation state of a molecule, whether it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate (chemistry), substrate, it is a subcategory of hydrolases. Phosphatase enzymes are essential to many biological functions, because phosphorylation (e.g. by protein kinases) and dephosphorylation (by phosphatases) serve diverse roles in cell growth, cellular regulation and cell signaling, signaling. Whereas phosphatases remove phosphate groups from molecules, kinases catalyze the transfer of phosphate groups to molecules from Adenosine triphosphate, ATP. Together, kinases and phosphatases direct a form of post-translational modification that is essential to the cell's regulatory network. Phosphatase enzymes are not to be confused with phosphorylase enzymes, which catalyze the transfer of a phosphate group from hydrogen phosphate to an acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |