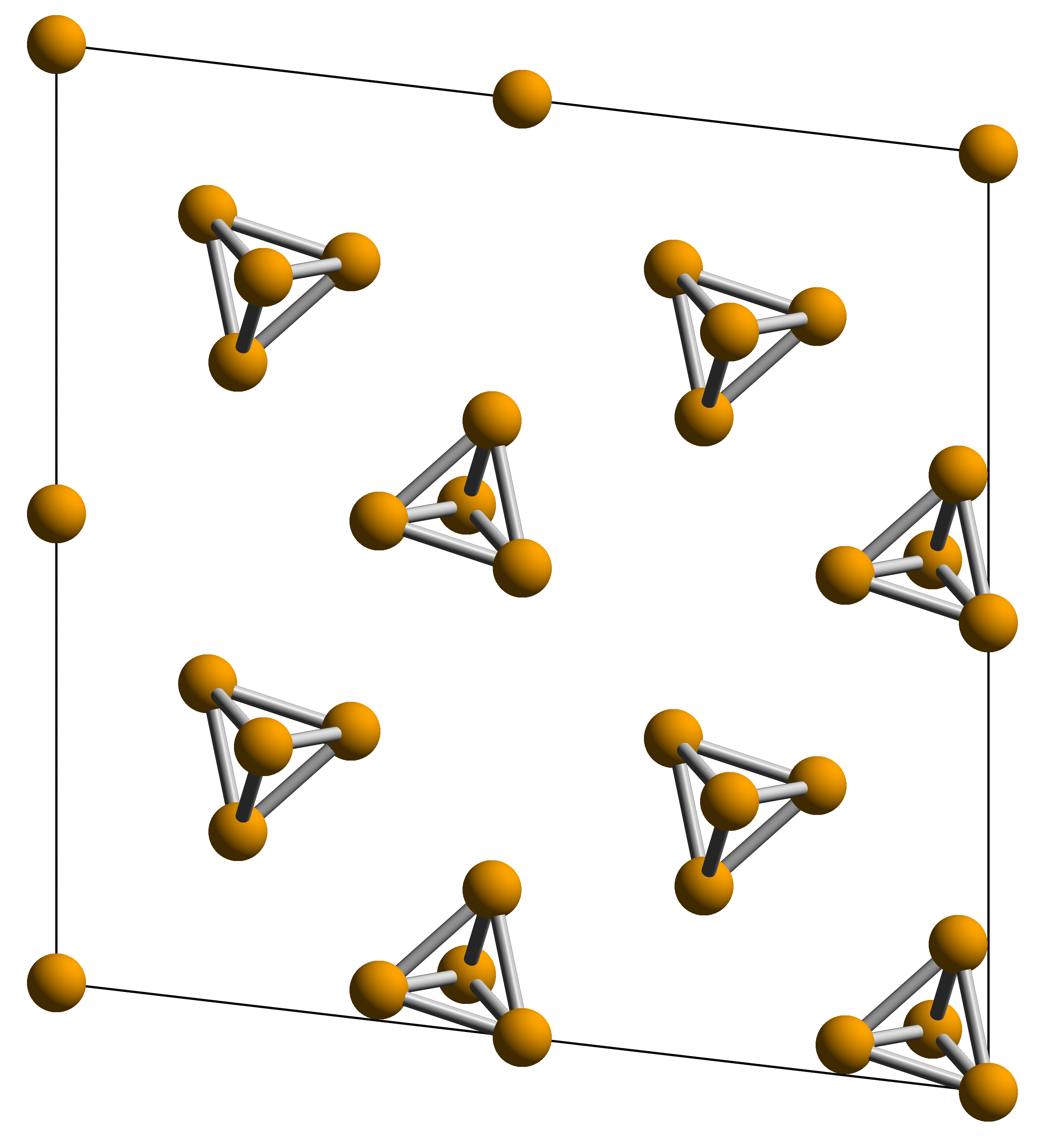|
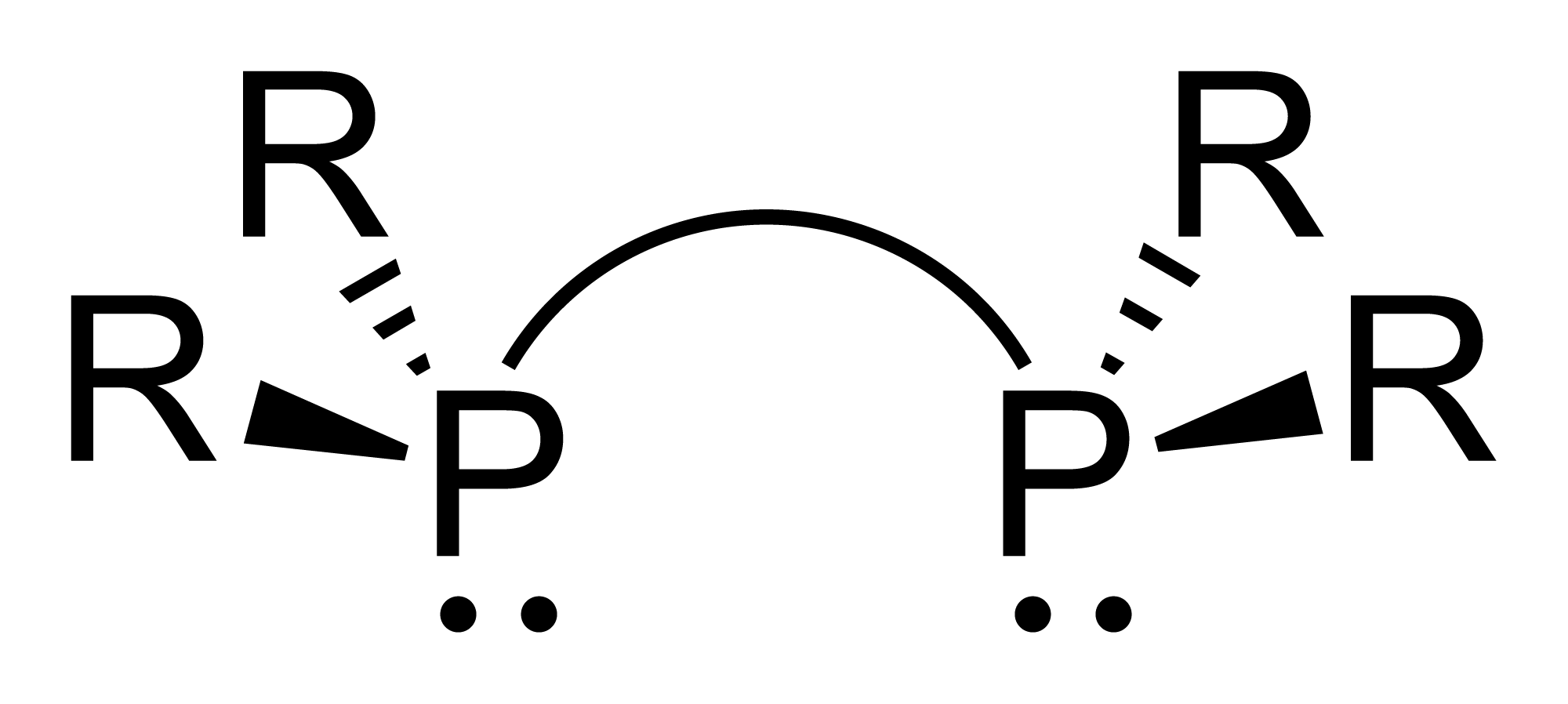
Phosphine Complexes
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but chemical purity, technical grade samples have a highly Odor#Types, unpleasant odor like rotting fish, due to the presence of substitution reaction, substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a Trigonal pyramidal molecular geometry, trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs to the ether class of organic compounds. It is a common solvent and was formerly used as a general anesthetic. Production Most diethyl ether is produced as a byproduct of the vapor-phase Hydration reaction, hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid Catalysis, catalysts and can be adjusted to make more ether if the need arises: Vapor-phase Dehydration reaction, dehydration of ethanol over some Aluminium oxide, alumina catalysts can give diethyl ether yields of up to 95%. : Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Uses The dominant use of diethyl ether is as a solvent. One particular application is in the production of cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substitution Reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent. A good example of a substitution reaction is halogenation. When chlorine gas (Cl2) is irradiated, some of the molecules are split into two chlorine radicals (Cl•), whose free electrons are stron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Phosphide
Calcium phosphide (CP) is the inorganic compound with the formula Ca3P2. It is one of several phosphides of calcium, being described as the salt-like material composed of Ca2+ and P3−. Other, more exotic calcium phosphides have the formula CaP / Ca2P2, CaP3, and Ca5P8. Ca3P2 has the appearance of red-brown crystalline powder or grey lumps. Its trade name is Photophor for the incendiary use or Polytanol for the use as rodenticide. Preparation, history and structure It may be formed by reaction of the elements, but it is more commonly prepared by carbothermal reduction of calcium phosphate: :Ca3(PO4)2 + 8 C → Ca3P2 + 8 CO This is also the way how it was accidentally discovered by Smithson Tennant in 1791 while verifying the composition of carbon dioxide proposed by Antoine Lavoisier by reducing calcium carbonate with phosphorus. The structure of the room temperature form of Ca3P2 has not been confirmed by X-ray crystallography. A high temperature phase h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cold Trap
In vacuum applications, a cold trap is a device that condenses all vapors except the permanent gases (hydrogen, oxygen, and nitrogen) into a liquid or solid. The most common objective is to prevent vapors being evacuated from an experiment from entering a vacuum pump where they would condense and contaminate it. Particularly large cold traps are necessary when removing large amounts of liquid as in freeze drying. Cold traps also refer to the application of cooled surfaces or baffles to prevent oil vapours from flowing from a pump and into a chamber. In such a case, a baffle or a section of pipe containing a number of cooled vanes, will be attached to the inlet of an existing pumping system. By cooling the baffle, either with a cryogen such as a dry ice mixture, or by use of an electrically driven Peltier element, oil vapour molecules that strike the baffle vanes will condense and thus be removed from the pumped cavity. Applications Pumps that use oil either as their working fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Louis Jacques Thénard
Louis Jacques Thénard (4 May 177721 June 1857) was a French chemist. Life He was born in a farm cottage near Nogent-sur-Seine in the Champagne district the son of a farm worker. In the post-Revolution French educational system, most boys received scholarships for education up to age 14, and this allowed him to be educated at the academy at Sens. He then went at the age of sixteen to study pharmacy in Paris. There he attended the lectures of Antoine François Fourcroy and Louis Nicolas Vauquelin. He was allowed into Vauquelin's laboratory even though he was unable to pay the monthly fee of 20 francs, due to the requests of Vauquelin's sisters. But his progress was so rapid that in two or three years he was able to take his master's place at the lecture-table, and Fourcroy and Vauquelin were so satisfied with his performance that they procured for him a school appointment in 1797 as a teacher of chemistry, and in 1798 one as at the École Polytechnique. Career In 1804 Vauquel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potash
Potash ( ) includes various mined and manufactured salts that contain potassium in water- soluble form.Potash , USGS 2008 Minerals Yearbook The name derives from ''pot ash'', plant ashes or soaked in water in a pot, the primary means of manufacturing potash before the Industrial Era. The word '''' is derived from ''potash''. Potash is produced worldwide in amounts exceeding 71.9 million |
White Phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white " diphosphorus pentoxide", which consists of tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), CNRS () also Antoine Lavoisier after the French Revolution, was a French nobleman and who was central to the 18th-century |
Triphosphane
Triphosphane (IUPAC systematic name) or triphosphine is an inorganic compound having the chemical formula . It can be generated from diphosphine but is highly unstable at room temperature: : Samples have been isolated by gas chromatography Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include t .... The compound rapidly converts to and the cyclophosphine ''cyclo''-. References External linksIUPAC {{Hydrides by group Phosphines Phosphorus hydrides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphine
Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphines (''n'' = 2), tertiary phosphines (''n'' = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3). Annette Schier and Hubert Schmidbaur"P-Donor Ligands" in Encyclopedia of Inorganic Chemistry 2006, Wiley-VCH, Weinheim. 1° vs 2° vs 3° phosphines Organophophines are classified according to the number of organic substituents. Primary phosphines Primary (1°) phosphines, with the formula RPH2, in principle are derived by alkylation of phosphine. Some simple alkyl derivatives such as methylphosphine (CH3PH2) can be prepared by alkylation of phosphine in the presence of base: : (M = Li, Na, K) A more common s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Pyramidal Molecular Geometry
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). When all three atoms at the corners are identical, the molecule belongs to point group ''C3v''. Some molecules and ions with trigonal pyramidal geometry are the pnictogen hydrides (XH3), xenon trioxide (XeO3), the chlorate ion, , and the sulfite ion, . In organic chemistry, molecules which have a trigonal pyramidal geometry are sometimes described as sp3 hybridized. The AXE method for VSEPR theory states that the classification is AX3E1. Trigonal pyramidal geometry in ammonia The nitrogen in ammonia has 5 valence electrons and bonds with three hydrogen atoms to complete the octet. This would result in the geometry of a regular tetrahedron with each bond angle equal to arccos(−) ≈ 109.5°. However, the three hydrogen atoms are repelled by the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |