|
Philips Catalyst
The Phillips catalyst, or the Phillips supported chromium catalyst, is the catalyst used to produce approximately half of the world's polyethylene. A heterogeneous catalyst, it consists of a chromium oxide catalyst support, supported on silica gel. Polyethylene, the most-produced synthetic polymer, is produced industrially by the polymerization of ethylene: :n C2H4 → (C2H4)n Although exergonic (i.e., thermodynamically favorable), the reaction requires catalysts. Three main catalysts are employed commercially: the Phillips catalyst, Ziegler–Natta catalysts (based on titanium trichloride), and, for specialty polymers, metallocene-based catalysts. Preparation and mechanism of action The Phillips catalyst is prepared by impregnating high surface area silica gel with chromium trioxide or related chromium compounds. The solid precatalyst is then calcined in air to give the active catalyst. Only a fraction of the chromium is catalytically active, a fact that interferes with el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium Trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula . It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen. Production, structure, and basic reactions Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: : Approximately 100,000 tonnes are produced annually by this or similar routes. The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3. The structure of monomeric has been ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complexes
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form reaction intermediate, intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous catalysis, homogeneou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Schulz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Processes
Industrial processes are procedures involving chemical, physical, electrical, or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry. Chemical processes by main basic material Certain chemical process yield important basic materials for society, e.g., (cement, steel, aluminum, and fertilizer). However, these chemical reactions contribute to climate change by emitting carbon dioxide, a greenhouse gas, through chemical reactions, as well as through the combustion of fossil fuels to generate the high temperatures needed to reach the activation energies of the chemical reactions. Cement (the paste within concrete) * Calcination – Limestone, which is largely composed of fossilized calcium carbonate (CaCO3), breaks down at high temperatures into useable calcium oxide (CaO) and carbon dioxide gas (), which gets released as a by-product. This chemical reaction, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phillips Petroleum
Phillips Petroleum Company was an American oil company incorporated in 1917 that expanded into petroleum refining, marketing and transportation, natural gas gathering and the chemicals sectors. It was Phillips Petroleum that first found oil in the North Sea on December 23, 1969, at a position that was later named Ekofisk. On August 30, 2002, Conoco Inc. merged with Phillips Petroleum to form ConocoPhillips, becoming the third largest integrated energy company and second-largest refining company in the United States. The company moved its headquarters to Houston.Christopher J. Castaneda,"Phillips Petroleum Company." ''Encyclopedia of Oklahoma History and Culture''.Accessed 04 February 2013. In 2012, ConocoPhillips split into two separate companies. The legacy company kept its name, and spun off the midstream and downstream portions of its business. The new company, which owns the refinery, chemical and pipeline assets formerly held in ConocoPhillips, is named Phillips 66, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Banks (chemist)
Robert L. Banks (November 24, 1921 – January 3, 1989) was an American chemist. He was born and grew up in Piedmont, Missouri. He attended the Missouri University of Science and Technology, University of Missouri, Rolla, and initiated into Alpha Phi Omega in 1940. He joined the Phillips Petroleum company in 1946 and worked there until he retired in 1985. He died in Missouri on January 3, 1989. Technical contributions He was a fellow research chemist of J. Paul Hogan. They began working together in 1946, and in 1951 invented "crystalline polypropylene" and high-density polyethylene (HDPE). These plastics were initially known by the name Marlex. The polymerization of ethylene was made possible by their discovery of the so-called Phillips catalyst. Recognition In 1987, Banks and Hogan won the Perkin Medal, and in 2001 they were inducted into the National Inventors Hall of Fame. Both were given a ''Heroes of Chemistry'' award by the American Chemical Society in 1989. Banks was induc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochromium Chemistry
Organochromium chemistry is a branch of organometallic chemistry that deals with organic compounds containing a chromium to carbon bond and their reactions. The field is of some relevance to organic synthesis. The relevant oxidation states for organochromium complexes encompass the entire range of possible oxidation states from –4 (d10) in Na4 r–IV(CO)4to +6 (d0) in oxo-alkyl complexes like Cp*CrVI(=O)2Me. History The first organochromium compound was described in 1919 by Franz Hein. He treated phenylmagnesium bromide with chromium(III) chloride to give a new product (after hydrolysis) which he incorrectly identified as pentaphenyl chromium bromide (Ph5CrBr). Years later, in 1957 H.H. Zeiss et al. repeated Hein's experiments and correctly arrived at a cationic bisarene chromium sandwich compound (ArH2Cr+). Bis(benzene)chromium itself was discovered around the same time in 1956 by Ernst Otto Fischer by reaction of chromium(III) chloride, benzene and aluminum chloride. The rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
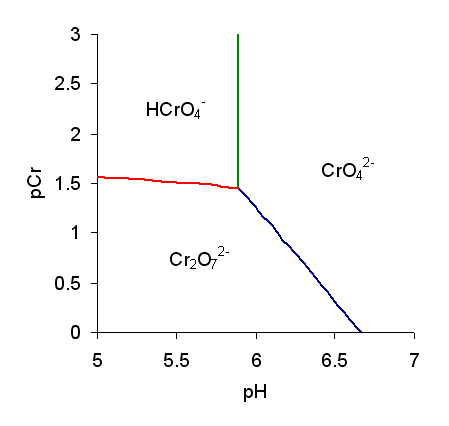
Chromate And Dichromate
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, Potassium chromate Potassium-dichromate-sample.jpg, Potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, , is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex . Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. : The predominance diagram shows that the position of the equilibrium depends on both pH and the analytical concentration o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcine
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (). A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled atmosphere. Etymology The process of calcination deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |