|
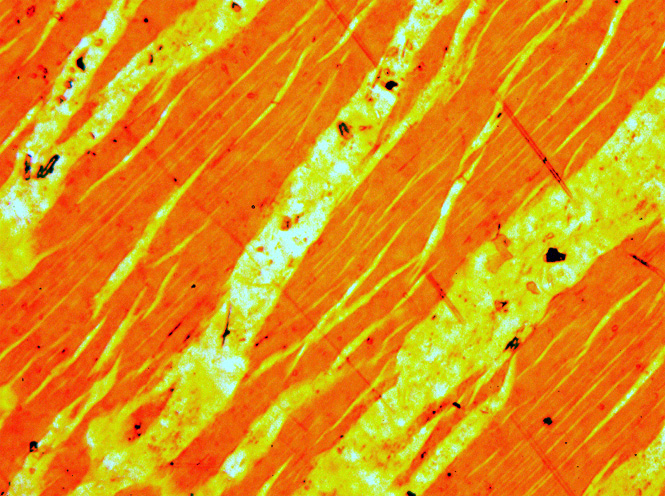
Perthite
Perthite or perthitic texture is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perthite Textures
Perthite or perthitic texture is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust and 41% of the Earth's continental crust by weight. Feldspars crystallize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Etymology The name ''feldspar'' derives from the German , a compound of the words ' ("field") and ("flake"). had long been used as the word for "a rock easily cleaved into flakes"; was introduced in the 18th century as a more specific term, referring perhaps to its comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust and 41% of the Earth's continental crust by weight. Feldspars crystallize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Etymology The name ''feldspar'' derives from the German , a compound of the words ' ("field") and ("flake"). had long been used as the word for "a rock easily cleaved into flakes"; was introduced in the 18th century as a more specific term, referring perhaps to its common occurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust and 41% of the Earth's continental crust by weight. Feldspars crystallize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Etymology The name ''feldspar'' derives from the German , a compound of the words ' ("field") and ("flake"). had long been used as the word for "a rock easily cleaved into flakes"; was introduced in the 18th century as a more specific term, referring perhaps to its comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcline
Microcline (KAlSi3O8) is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during slow cooling of orthoclase; it is more stable at lower temperatures than orthoclase. Sanidine is a polymorph of alkali feldspar stable at yet higher temperature. Microcline may be clear, white, pale-yellow, brick-red, or green; it is generally characterized by cross-hatch twinning that forms as a result of the transformation of monoclinic orthoclase into triclinic microcline. The chemical compound name is potassium aluminium silicate, and it is known as E number reference E555. Geology Microcline may be chemically the same as monoclinic orthoclase, but because it belongs to the triclinic crystal system, the prism angle is slightly less than right angles; hence the name "microcline" from the Greek "small slope". It is a fully ordered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create cation, an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-flame color, colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamella (materials)
A lamella (: lamellae) is a small plate or flake, from the Latin, and may also refer to collections of fine sheets of material held adjacent to one another in a gill-shaped structure, often with fluid in between though sometimes simply a set of "welded" plates. The term is used in biological contexts for thin membranes of plates of tissue. In the context of materials science, the microscopic structures in bone and nacre are called lamellae. Moreover, the term lamella is often used to describe crystal structure of some materials. Uses of the term In surface chemistry (especially mineralogy and materials science), lamellar structures are fine layers, alternating between different materials. They can be produced by chemical effects (as in eutectic solidification), biological means, or a deliberate process of lamination, such as pattern welding. Lamellae can also describe the layers of atoms in the crystal lattices of materials such as metals. In surface anatomy, a lamella is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. It represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula . It is a tectosilicate. Its color is usually pure white, hence its name from Latin, . It is a common constituent in felsic rocks. Properties Albite crystallizes with triclinic pinacoidal forms. Its specific gravity is about 2.62 and it has a Mohs hardness of 6 to 6.5. Albite almost always exhibits crystal twinning often as minute parallel striations on the crystal face. Albite often occurs as fine parallel segregations alternating with pink microcline in perthite as a result of exolution on cooling. There are two variants of albite, which are referred to as 'low albite' and 'high albite'; the latter is also known as 'analbite'. Although both variants are triclinic, they differ in the volume of their unit cell, which is slightly larger for the 'high' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoclase
Orthoclase, or orthoclase feldspar ( endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture", because its two cleavage planes are at right angles to each other. It is a type of alkali feldspar, also known as potassium feldspar or K-spar. The gem known as moonstone (see below) is largely composed of orthoclase. Formation and subtypes left, Orthoclase Organ_Mountains.html" ;"title="crystal twinning from the Organ Mountains">crystal twinning from the Organ Mountains in New Mexico Orthoclase is a common constituent of most granites and other felsic igneous rocks and often forms huge crystals and masses in pegmatite. Typically, the pure potassium endmember of orthoclase forms a solid solution with albite, the sodium endmember (NaAlSi3O8) of plagioclase. While slowly cooling within the earth, sodium-rich albite lamellae form by exsolution, enriching the remaining orthocla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exsolution
A solid solution, a term popularly used for metals, is a homogeneous mixture of two compounds in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions – ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under the brand na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Solution
A solid solution, a term popularly used for metals, is a homogeneous mixture of two compounds in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions – ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under the bra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |