|
Perfluoropolyether
Perfluoropolyethers (PFPEs) are a class of organofluorine compound. Some types are synthetic liquid lubricants that have been used in the aerospace industry for over 30 years. The main properties of PFPE are being temperature resistant between and (depending on specific composites), having very low outgassing compared to other fluids (vapour pressure of ) and having a dielectric strength of around 15.7 MV/m. Perfluoropolyethers consists of a polymer chain in which monomers consisting of perfluoro-alkyl groups are joined by ether linkages. The bonds between carbon and oxygen or fluorine are strong. Perfluoropolyethers are a type of PFAS. The thermal and chemical stability of PFPEs along with a vapor–liquid equilibrium of 230 °C when mixed with the right composites make it a suitable candidate for vapor phase soldering technologies. History Perfluoropolyethers were developed in the early 1960s for the USAF, who needed a lubricant that would not react with liquid or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Compound
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). This is significantly st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HFPO
Hexafluoropropylene oxide (HFPO) is an intermediate used in industrial organofluorine chemistry; specifically it is a monomer for fluoropolymers. This colourless gas is the epoxide of hexafluoropropylene, which is a fluorinated analog of propylene oxide, HFPO is produced by Chemours and 3M and as a precursor to the lubricant Krytox and related materials. It is generated by oxidation of perfluoropropylene, e.g. with oxygen as well as other oxidants. Reactivity Fluoride catalyzes the formation of perfluorinated polyethers such as Krytox. The initial step entails nucleophilic attack at the middle carbon to give the perfluoropropoxide anion, which in turn attacks another monomer. This process generates a polymer terminated by an acyl fluoride, which is hydrolyzed to the carboxylic acid which is decarboxylated with fluorine. The net polymerization reaction can be represented as: : Upon heating above 150 °C, HFPO decomposes to trifluoroacetyl fluoride and difluorocarbene: : The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plug Valve
Plug valves are valves with cylindrical or conically tapered "plugs" which can be rotated inside the valve body to control flow through the valve. The plugs in plug valves have one or more hollow passageways going sideways through the plug, so that fluid can flow through the plug when the valve is open. Plug valves are simple and often economical. When the plug is conically tapered, the stem/handle is typically attached to the larger diameter end of the plug. Plug valves usually do not have bonnets but often have the end of the plug with the handle exposed or mostly exposed to the outside. In such cases, there is usually not much of a stem. The stem and handle often come in one piece, often a simple, approximately L-shaped handle attached to the end of the plug. The other end of the plug is often exposed to the outside of the valve too, but with a mechanism that retains the plug in the body. The simplest and most common general type of plug valve is a 2-port valve with two posit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine Gas
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to sulfuric acid. Sulfur trioxide exists in several forms: gaseous monomer, crystalline trimer, and solid polymer. Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain. Molecular structure and bonding Monomer The molecule SO3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). When the formal charge is non-zero, the S-O bonding is assumed to be delocalized. In any case the three S-O bond leng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid). Oleums can be described by the formula ''y''SO3·H2O where ''y'' is the total molar mass of sulfur trioxide content. The value of ''y'' can be varied, to include different oleums. They can also be described by the formula H2SO4·''x''SO3 where ''x'' is now defined as the molar free sulfur trioxide content. Oleum is generally assessed according to the free SO3 content by mass. It can also be expressed as a percentage of sulfuric acid strength; for oleum concentrations, that would be over 100%. For example, 10% oleum can also be expressed as H2SO4·''0.13611''SO3, ''1.13611''SO3·H2O or 102.25% sulfuric acid. The conversion between % acid and % oleum is: :\%\,\text = 100 + \frac \times \%\,\text For ''x'' = 1 and ''y'' = 2 the empirical formul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylene Glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH. PEG is commonly incorporated into hydrogels which present a functional form for further use. Uses Medical uses * Pharmaceutical-grade PEG is used as an excipient in many pharmaceutical products, in oral, topical, and parenteral dosage forms. * PEG is the basis of a number of laxatives (as ''MiraLax, RestoraLAX, MoviPrep, etc.''). Whole bowel irrigation with polyethylene glycol and added electrolytes is used for bowel preparation before surgery or colonoscopy or for children with constipation. Macrogol (with brand names such as Laxido, Movicol and Miralax) is the generic name for polyethylene glycol used as a laxative. The name may be followed by a number th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterification
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid, sulfu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2,3,3-tetrafluorooxetane
The comma is a punctuation mark that appears in several variants in different languages. Some typefaces render it as a small line, slightly curved or straight, but inclined from the vertical; others give it the appearance of a miniature filled-in figure placed on the baseline. In many typefaces it is the same shape as an apostrophe or single closing quotation mark . The comma is used in many contexts and languages, mainly to separate parts of a sentence such as clauses, and items in lists mainly when there are three or more items listed. The word ''comma'' comes from the Greek (), which originally meant a cut-off piece, specifically in grammar, a short clause. A comma-shaped mark is used as a diacritic in several writing systems and is considered distinct from the cedilla. In Byzantine and modern copies of Ancient Greek, the " rough" and "smooth breathings" () appear above the letter. In Latvian, Romanian, and Livonian, the comma diacritic appears below the letter, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
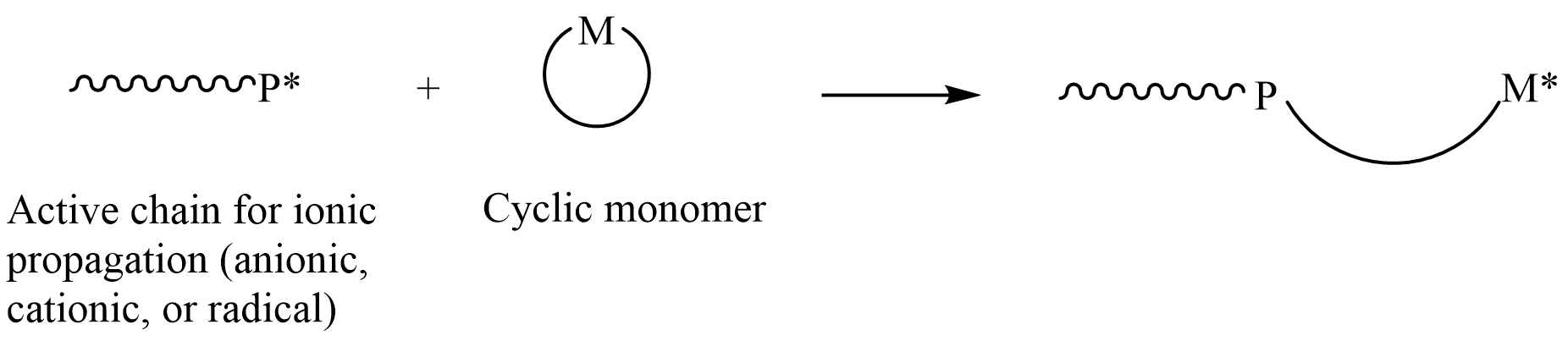
Ring-opening Polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anionic or cationic. Ring-opening of cyclic monomers is often driven by the relief of bond-angle strain. Thus, as is the case for other types of polymerization, the enthalpy change in ring-opening is negative. Many rings undergo ROP. Monomers Many cyclic monomers are amenable to ROP. These include epoxides, cyclic trisiloxanes, some lactones and lactides, cyclic anhydrides, cyclic carbonates, and amino acid ''N''-carboxyanhydrides. Many strained cycloalkenes, e.g norbornene, are suitable monomers via ring-opening metathesis polymerization. Even highly strained cycloalkane rings, such as cyclopropane and cyclobutane derivatives, can undergo ROP. History Ring-opening polymerization has been used since the beginning of the 1900s to produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroethylene
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula . It is a colorless gas. Its structure is . It is used primarily in the industrial preparation of fluoropolymers. It is the simplest perfluorinated alkene. It was first reported as "dicarbon tetrafluoride" in 1890. Properties Tetrafluoroethylene is a synthetic colorless, odorless gas that is insoluble in water. Like all unsaturated fluorocarbons, it is susceptible to nucleophilic attack. It is unstable towards decomposition to carbon and carbon tetrafluoride () and prone to form explosive peroxides in contact with air. Industrial use Polymerization of tetrafluoroethylene produces polytetrafluoroethylene (PTFE) polymers such as Teflon and Fluon. PTFE is one of the two fluorocarbon resins composed wholly of fluorine and carbon. The other resin composed purely of carbon and fluorine is the copolymer of TFE with typically 6–9% hexafluoropropene (HFP), which is known as FEP (fluorinated ethylene propylene c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |