|
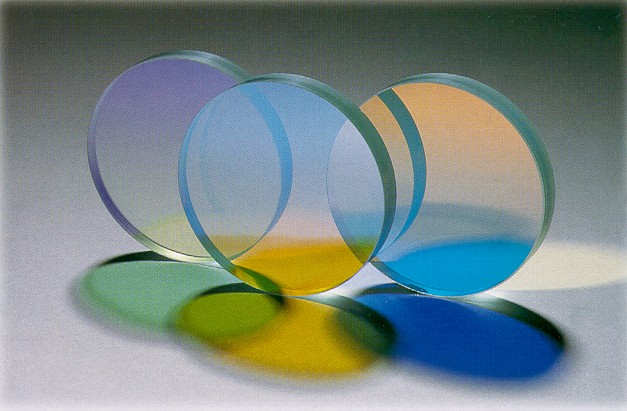
Perfluoroalkoxy Alkane
Perfluoroalkoxy alkanes (PFA) are fluoropolymers. They are copolymers of tetrafluoroethylene (C2F4) and perfluoroethers (C2F3ORf, where Rf is a perfluorinated group such as trifluoromethyl (CF3)). The properties of these polymers are similar to those of polytetrafluoroethylene (PTFE). Compared to PTFE, PFA has better anti-stick properties and higher chemical resistance, at the expense of lesser scratch resistance. Properties Unlike with PTFE, the alkoxy substituents allow the polymer to be melt-processed. On a molecular level, PFA polymers have a smaller chain length and higher chain entanglement than other fluoropolymers. They also contain an oxygen atom at the branches. This results in materials that are more translucent and have improved flow and creep resistance, with thermal stability close to or exceeding PTFE. Thus, PFA is preferred when extended service is required in hostile environments involving chemical, thermal, and mechanical stress. PFA offers high melt strength ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoroether
Perfluoroethers are a class of organofluorine compound containing one or more ether functional group. In general these compounds are structurally analogous to the related hydrocarbon ethers, except for the distinctive properties of fluorocarbons. The introduction of an ether function to a perfluoro-polymer chain also provides thermoplastic properties to the polymer, making thermal forming possible. This is a great technological advantage for producing a large variety of shapes (e.g., beakers, funnels, flasks for laboratory uses, etc...) and allows extrusion of highly chemically-resistant tubing. It also confers on the polymer a translucent appearance. Low molecular weight fluorinated ethers Acyclic perfluoroethers are analogues of diethylether, e.g. O(C2F5)2, such perfluoro(2-ethoxyethane)sulfonic acid (PFEESA). Of practical utility are the fluorinated epoxides. Tetrafluoroethylene oxide and hexafluoropropylene oxide. These are precursors of perfluoro(methyl vinyl ether) ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company. History In 1938, polytetrafluoroethylene (DuPont brand name Teflon) was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with tetrafluoroethylene gas to develop refrigerants, he noticed that a previously pressurized cylinder had no pressure remaining. In dissecting the cylinder, he found a mass of white solid in a quantity similar to that of the tetrafluoroethylene gas. It was determined that this material was a new-to-the-world polymer. Tests showed the substance was resistant to corrosion from most acids, bases and solvents and had better high temperature stability than any other plastic. By early 1941, a crash program was making substantial quantities of PT ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroalcohol
Fluoroalcohols are organofluorine compounds consisting of an alcohol functional group with at least one C-F bond. These compounds often have distinctive solvent properties. Perfluoroalcohols Most primary and secondary perfluoroalcohols are unstable, for example trifluoromethanol eliminates hydrogen fluoride, forming carbonyl fluoride. This reaction is reversible. : Stable perfluorinated alcohols include nonafluoro-tert-butyl alcohol ((CF3)3COH) and pentafluorophenol (C6F5OH). Partially fluorinated alcohols Numerous partially fluorinated alcohols are known and have useable stabilities. Trifluoroethanol and hexafluoroisopropanol are used as solvents in research.{{cite journal , doi=10.1038/s41570-017-0088, title=Hexafluoroisopropanol as a Highly Versatile Solvent , year=2017 , last1=Colomer , first1=Ignacio , last2=Chamberlain , first2=Anna E. R. , last3=Haughey , first3=Maxwell B. , last4=Donohoe , first4=Timothy J. , journal=Nature Reviews Chemistry , volume=1 , issu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis.excerpt Transition metal alkoxides are widely used for coatings and as catalysts. Enolates are unsaturated alkoxides derived by deprotonation of a bond adjacent to a ketone or aldehyde. The nucleophilic center for simple alkoxides is located on the oxygen, whereas the nucleophilic site on enolates is delocalized onto both carbon and oxygen sites. Ynolates are also unsaturated alkoxides derived from acetylenic alcohols. Phenoxides are close relatives of the alkoxides, in which the alkyl group is replaced by a phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoro(methyl Vinyl Ether)
Perfluoro(methyl vinyl ether) is a perfluorinated compound used as a precursor to fluoropolymers. It is the simplest unsaturated perfluoroether. Preparation Preparation begins with hexafluoropropylene oxide (HFPO) and carbonyl fluoride over a metal fluoride catalyst. The fluoride reacts with the carbonyl, forming a perfluoroalkoxide anion. The anion attacks the electrophilic central carbon atom of HFPO in a nucleophilic ring-opening reaction similar to anionic polymerization. Elimination of fluoride regenerates the catalyst and yields perfluoro(2-methoxy propionyl fluoride). The acyl fluoride is then treated with potassium hydroxide to produce the perfluorocarboxylate. The carboxylate is then heated, causing decarboxylation followed by elimination of fluoride Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfa Tubing
PFA or Pfa may refer to: Organizations * Pacific Film Archive, now part of the Berkeley Art Museum and Pacific Film Archive (BAM/PFA) * People for Animals, Indian animal welfare organisation * PFA Footcare Association (Canadian Chapter) * Police Federation of Australia * Policía Federal Argentina, the Argentine Federal Police * Popular Flying Association, a former name of the Light Aircraft Association * Popular Front of Azerbaijan, forerunner of the Popular Front Party of Azerbaijan (PFPA) * Professional Fraternity Association Science, technology, and mathematics Chemistry and materials science * Paraformaldehyde, a fixation solution * Perfluoroalkoxy alkane, a plastic or polymer resin * Pulverised fuel ash, a waste product of pulverised coal * Parafluoroamphetamine * Performic acid * See also PFAS, Per- and polyfluoroalkyl substances Computer science * Probabilistic finite automaton * .pfa, ''Printer Font ASCII'', a file extension for PostScript Printer Font ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoropropylene
Hexafluoropropylene is the fluoroalkene with the formula CF3CF=CF2. It is the perfluorocarbon counterpart to the hydrocarbon propylene. It is mainly used to produce copolymers with tetrafluoroethylene. Hexafluoropropylene is used as a chemical intermediate. Preparation Hexafluoropropylene can be produced by pyrolysis of tetrafluoroethylene Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula . It is a colorless gas. Its structure is . It is used primarily in the industrial preparation of fluoropolymers. It is the simplest perfluorinated alkene. It was first repor ...: :3CF2=CF2 → 2CF3CF=CF2 It can also be prepared from chlorodifluoromethane, or produced from various chlorofluorocarbons. References Perfluoroalkenes {{organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorinated Ethylene Propylene
Fluorinated ethylene propylene (FEP) is a copolymer of hexafluoropropylene and tetrafluoroethylene. It differs from the polytetrafluoroethylene (PTFE) resins in that it is melt-processable using conventional injection molding and Plastic extrusion, screw extrusion techniques. Fluorinated ethylene propylene was invented by DuPont and is sold under the brandname Teflon FEP. Other brandnames are Neoflon FEP from Daikin or Dyneon FEP from Dyneon/3M. FEP is very similar in composition to the fluoropolymers polytetrafluoroethylene, PTFE (polytetrafluoroethylene) and Perfluoroalkoxy polymer resin, PFA (perfluoroalkoxy polymer resin). FEP and PFA both share PTFE's useful properties of low friction and non-reactivity, but are more easily formable. FEP is softer than PTFE and melts at 260 °C; it is highly transparent and resistant to sunlight. Production FEP is produced by free-radical polymerization of mixtures of tetrafluoroethylene and hexafluoropropylene. The mixture is biased to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transparency And Translucency
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) is the physical property of allowing light to pass through the material (with or without scattering of light). It allows light to pass through but the light does not necessarily follow Snell's law on the macroscopic scale; the photons may be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxy
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also known as ethoxybenzene). Related to alkoxy groups are aryloxy groups, which have an aryl group singularly bonded to oxygen such as the phenoxy group (). An alkoxy or aryloxy group bonded to an alkyl or aryl () is an ether. If bonded to H it is an Alcohol (chemistry), alcohol. The term ''alkoxide'' refers to the anionic conjugate bases of alcohols () or to ionic compounds containing such an anion. Alkoxide compounds are derivatives of alcohols where the hydrogen of the –OH group is replaced by a metal; for example, the sodium salt (chemistry), salt of ethanol () is sodium ethoxide, containing ethoxide anions and sodium cations . References {{DEFAULTSORT:Alkoxy Group Alkoxy groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a corporate spin-off, spin-off from DuPont (1802–2017), DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances Wetting, wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low polarizability, electric polarizability of fluorine. PTFE has one of the lowest Friction#Coefficient of friction, coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for Cookware and bakeware, pans and other Cookware and bakeware, cookware. It is Chemically inert, non-reactive, partly because of the strength of car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethyl
The trifluoromethyl group is a functional group that has the formula . The naming of is group is derived from the methyl group (which has the formula ), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane , 1,1,1-trifluoroethane , and hexafluoroacetone . Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from 1928, although re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |