|
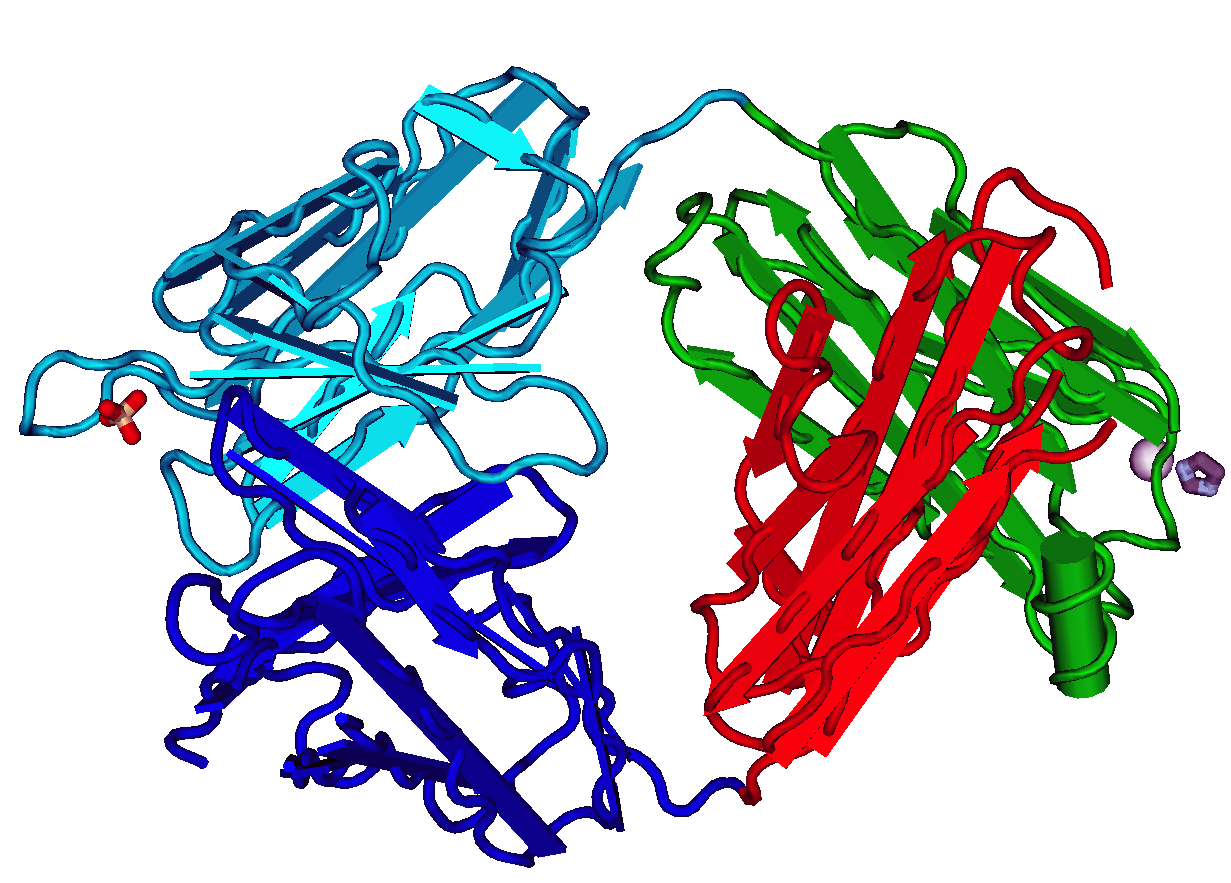
Paratope
In immunology, a paratope, also known as an antigen-binding site, is the part of an antibody which recognizes and binds to an antigen. It is a small region at the tip of the antibody's antigen-binding fragment and contains parts of the antibody's heavy and light chains. Each paratope is made up of six complementarity-determining regions - three from each of the light and heavy chains - that extend from a fold of anti-parallel beta sheets. Each arm of the Y-shaped antibody has an identical paratope at the end. Paratopes make up the parts of the B-cell receptor that bind to and make contact with the epitope of an antigen. All the B-cell receptors on any one individual B cell have identical paratopes. The uniqueness of a paratope allows it to bind to only one epitope with high affinity and as a result, each B cell can only respond to one epitope. The paratopes on B-cell receptors binding to their specific epitope is a critical step in the adaptive immune response. Design of par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Basic Unit
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epitope
An epitope, also known as antigenic determinant, is the part of an antigen that is recognized by the immune system, specifically by antibodies, B cells, or T cells. The part of an antibody that binds to the epitope is called a paratope. Although epitopes are usually non-self proteins, sequences derived from the host that can be recognized (as in the case of autoimmune diseases) are also epitopes. The epitopes of protein antigens are divided into two categories, conformational epitopes and linear epitopes, based on their structure and interaction with the paratope. Conformational and linear epitopes interact with the paratope based on the 3-D conformation adopted by the epitope, which is determined by the surface features of the involved epitope residues and the shape or tertiary structure of other segments of the antigen. A conformational epitope is formed by the 3-D conformation adopted by the interaction of discontiguous amino acid residues. In contrast, a linear epitope i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. Antigens can be proteins, peptides (amino acid chains), polysaccharides (chains of simple sugars), lipids, or nucleic acids. Antigens exist on normal cells, cancer cells, parasites, viruses, fungus, fungi, and bacteria. Antigens are recognized by antigen receptors, including antibodies and T-cell receptors. Diverse antigen receptors are made by cells of the immune system so that each cell has a specificity for a single antigen. Upon exposure to an antigen, only the lymphocytes that recognize that antigen are activated and expanded, a process known as clonal selection. In most cases, antibodies are ''antigen-specific'', meaning that an antibody can only react to and bind one specific antigen; in some instances, however, antibodies may cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragment Antigen-binding
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen. Preparation In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. Conversely, the enzyme pepsin cleaves below the hinge region, so the result instead is a F(ab')2 fragment and a pFc' fragment. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from ''Streptococcus pyogenes'', trade name FabRICATOR) cleaves IgG in a sequence specific manner a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
B-cell Receptor
The B-cell receptor (BCR) is a transmembrane protein on the surface of a B cell. A B-cell receptor is composed of a membrane-bound immunoglobulin molecule and a signal transduction moiety. The former forms a type 1 Transmembrane protein, transmembrane receptor protein, and is typically located on the Cell membrane, outer surface of these lymphocyte cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of the B cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B cells' mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR–antigen bonds directly. Particularly, grouping and spreading increase the relation of anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
V(D)J Recombination
V(D)J recombination (variable–diversity–joining rearrangement) is the mechanism of somatic recombination that occurs only in developing lymphocytes during the early stages of T and B cell maturation. It results in the highly diverse repertoire of Antibody, antibodies/immunoglobulins and T cell receptors (TCRs) found in B cells and T cells, respectively. The process is a defining feature of the adaptive immune system. V(D)J recombination in mammals occurs in the primary lymphoid organs (bone marrow for B cells and thymus for T cells) and in a nearly random fashion rearranges variable (V), joining (J), and in some cases, diversity (D) gene segments. The process ultimately results in novel amino acid sequences in the antigen-binding regions of immunoglobulins and TCRs that allow for the recognition of antigens from nearly all pathogens including bacteria, viruses, parasites, and Helminths, worms as well as "altered self cells" as seen in cancer. The recognition can also be Allergy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunology
Immunology is a branch of biology and medicine that covers the study of Immune system, immune systems in all Organism, organisms. Immunology charts, measures, and contextualizes the Physiology, physiological functioning of the immune system in states of both health and diseases; malfunctions of the immune system in immunological disorders (such as Autoimmune disease, autoimmune diseases, Hypersensitivity, hypersensitivities, immune deficiency, and transplant rejection); and the physical, chemical, and physiological characteristics of the components of the immune system ''in vitro'', ''In situ#Biology and biomedical engineering, in situ'', and ''in vivo''. Immunology has applications in numerous disciplines of medicine, particularly in the fields of organ transplantation, oncology, rheumatology, virology, bacteriology, parasitology, psychiatry, and dermatology. The term was coined by Russian biologist Ilya Ilyich Mechnikov, who advanced studies on immunology and received the Nob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Heavy Chain
The immunoglobulin heavy chain (IgH) is the large polypeptide subunit of an antibody (immunoglobulin). In the human genome, the IgH gene loci are on chromosome 14. A typical antibody is composed of two immunoglobulin (Ig) heavy chains and two Ig light chains. Several different types of heavy chain exist that define the class or isotype of an antibody. These heavy chain types vary between different animals. All heavy chains contain a series of immunoglobulin domains, usually with one variable domain (VH) that is important for binding antigen and several constant domains (CH1, CH2, etc.). Production of a viable heavy chain is a key step in B cell maturation. If the heavy chain is able to bind to a surrogate light chain and move to the plasma membrane, then the developing B cell can begin producing its light chain. The heavy chain does not always have to bind to a light chain. Pre-B lymphocytes can synthesize heavy chain in the absence of light chain, which then can allow the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Light Chain
] The immunoglobulin light chain is the small Peptide, polypeptide subunit of an antibody (immunoglobulin). A typical antibody is composed of two immunoglobulin (Ig) heavy chains and two Ig light chains. In humans There are two types of light chain in humans: * kappa (κ) chain, encoded by the immunoglobulin kappa locus (IGK@) on chromosome 2 (locus: 2p11.2) * lambda (λ) chain, encoded by the immunoglobulin lambda locus (IGL@) on chromosome 22 (locus: 22q11.2) Antibodies are produced by B lymphocytes, each expressing only one class of light chain. Once set, light chain class remains fixed for the life of the B lymphocyte. In a healthy individual, the total kappa-to-lambda ratio is roughly 2:1 in serum (measuring intact whole antibodies) or 1:1.5 if measuring free light chains, with a highly divergent ratio indicative of neoplasm. The free light chain ratio ranges from 0.26 to 1.65. Both the kappa and the lambda chains can increase proportionately, maintaining a normal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, Alzheimer's disease and other proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |