|
PFNA
Perfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also a persistent organic pollutant. Chemistry and properties In acidic form it is a highly reactive strong acid. In its conjugate base form as a salt (chemistry), salt it is stable and commonly ion paired with ammonium. In the commercial product Surflon S-111 (CAS 72968-3-88) it is the primary compound present by weight. PFNA is used as surfactant for the production of the fluoropolymer polyvinylidene fluoride.Supporting Information (PDF) It is produced mainly in Japan by the oxidation of a linear fluorotelomer olefin mixture containing F(CF2)8CH=CH2. It can also be synthesized by the carboxylation of F(CF2)8I. PFNA can form from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorosurfactant
Per- and polyfluoroalkyl substances (also PFAS, PFASs, and informally referred to as "forever chemicals") are a group of synthetic Organofluorine chemistry, organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain; there are 7 million known such chemicals according to PubChem. PFAS came into use with the invention of Teflon in 1938 to make fluoropolymer coatings and products that resist heat, oil, stains, grease, and water. They are now used in products including waterproof fabric such as Nylon, yoga pants, carpets, shampoo, feminine hygiene products, mobile phone screens, wall paint, furniture, adhesives, food packaging, firefighting foam, and the insulation of electrical wire. PFAS are also used by the cosmetic industry in most cosmetics and personal care products, including lipstick, eye liner, mascara, foundation (cosmetics), foundation, concealer, lip balm, blush (cosmetics), blush, and nail polish. Many PFAS such as Perfluorooctanesulfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PFOA
Perfluorooctanoic acid (PFOA; conjugate base perfluorooctanoate; also known colloquially as C8, from its chemical formula C8HF15O2) is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a chemical precursor. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, ''n''-heptyl "tail group" and a carboxylic acid "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic. The International Agency for Research on Cancer (IARC) has classified PFOA as carcinogenic to humans. PFOA is one of many synthetic organofluorine compounds collectively known as per- and polyfluoroalkyl substances (PFASs). Many PFAS such as PFOS, PFOA are a concern because they do not break down via natural processes and are commonly described as persistent organic pollutants or "forever chemicals". They can also m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorooctanoic Acid
Perfluorooctanoic acid (PFOA; conjugate acid, conjugate base perfluorooctanoate; also known colloquially as C8, from its chemical formula C8HF15O2) is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a chemical precursor. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, ''n''-heptyl "tail group" and a carboxylic acid "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic. The International Agency for Research on Cancer (IARC) has classified PFOA as carcinogenic to humans. PFOA is one of many synthetic organofluorine compounds collectively known as per- and polyfluoroalkyl substances (PFASs). Many PFAS such as Perfluorooctanesulfonic acid, PFOS, PFOA are a concern because they do not break down via natural processes and are commonly described as persistent organic pollutants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorotelomer Alcohol
Fluorotelomer alcohols, or FTOHs, are fluorotelomers with an alcohol functional group. They are volatile precursors to perfluorinated carboxylic acids, such as PFOA and PFNA, and other compounds. Naming Commonly, an individual fluorotelomer alcohol molecule is named by the number of carbons that are fluorinated versus the number that are hydrocarbon-based. For example, 8:2 fluorotelomer alcohol would represent a molecule with 8 fluorinated carbons and a 2 carbon ethyl alcohol group. The structure of a fluorotelomer alcohol is most commonly F(CF2)nCH2CH2OH, where ''n'' is an even number. Chemistry The synthesis of fluorotelomer alcohols requires a varying number of tetrafluoroethylene monomers that form an oligomer with a pentafluoroethyl iodide telogen. The fluorinated iodide then undergoes an addition with ethylene to form an organoiodine compound with increased synthesis possibilities. The terminal iodine is replaced by a hydroxyl group to yield the fluorotelomer alcohol. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorooctanesulfonic Acid
Perfluorooctanesulfonic acid (PFOS) (conjugate acid, conjugate base perfluorooctanesulfonate) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group, and thus it is a perfluorosulfonic acid and a Per- and polyfluoroalkyl substances, perfluoroalkyl substance (PFAS). It is an Human impact on the environment, anthropogenic (man-made) fluorosurfactant, now regarded as a global pollutant. PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and related stain repellents. The acronym "PFOS" refers to the parent sulfonic acid and to various Salt (chemistry), salts of perfluorooctanesulfonate. These are all colorless or white, water-soluble solids. Although of low acute toxicity, PFOS has attracted much attention for its pervasiveness and environmental impact. It was added to Annex B of the Stockholm Convention on Persistent Organic Pollutants in May 2009. History In 1949, 3M began producing PFOS-based compounds by electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surflon S-111
Surflon S-111 (CAS 72968-3-88) is a commercial product consisting of perfluorinated carboxylic acids (PFCAs) in ammonium salt form. It is commonly used as a polymerization aid in the production of fluoropolymers. The dominant chemical compound is perfluorononanoic acid (PFNA) at 74% by weight, followed by the 11 carbon perfluoroundecanoic acid (20%), and the 13 carbon perfluorotridecanoic acid (5%).Supporting Information (PDF). Surflon S-111 is synthesized in Japan by oxidizing a mixture of s. Fluorotelomer olefins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorotelomer
Fluorotelomers are fluorocarbon-based oligomers, or telomers, synthesized by telomerization. Some fluorotelomers and fluorotelomer-based compounds are a source of environmentally persistent perfluorinated carboxylic acids such as PFOA and PFNA, while others are under extended investigation. Types There are many broad categories of fluorotelomers: * Fluorotelomer alcohols (FTOH) * Fluorotelomer ethoxylates (FTEO) * Fluorotelomer fumarates * Fluorotelomer methacrylates (FTMAC) * Fluorotelomer sulphonates (FTS) Production In the radical telomerization of fluorotelomer molecules, a variety of fluorinated alkenes can serve as unsaturated taxogens including tetrafluoroethylene, vinylidene fluoride, chlorotrifluoroethylene, and hexafluoropropene. However, many fluorotelomers, such as fluorotelomer alcohols, are fluorocarbon-based because they are synthesized from tetrafluoroethylene. In addition to alcohols, synthetic products include fluorotelomer iodides, olefins, and acryla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorinated Carboxylic Acid
Perfluoroalkyl carboxylic acids (PFCAs), or perfluorocarboxylic acids are compounds of the formula CnF(2n+1)CO2H that belong to the class of per- and polyfluoroalkyl substances. The simplest example is trifluoroacetic acid. These compounds are organofluorine analogues of ordinary carboxylic acids, but they are stronger by several pKa units and they exhibit great hydrophobic character. Perfluoroalkyl dicarboxylic acids (PFdiCAs) are also known, e.g. C2F4(CO2H)2. Applications Trifluoroacetic acid is a widely employed acid, used for example in the synthesis of peptides. Its esters are useful in analytical chemistry. Longer-chain perfluoroalkyl carboxylic acids, e.g. with five to nine carbons, are useful fluorosurfactants and emulsifiers used in the production of polytetrafluoroethylene (Teflon) and related fluoropolymers. Production These compounds are typically prepared by electrochemical fluorination of the carboxylic acid fluorides followed by hydrolysis: :CnH(2n+1)COF ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
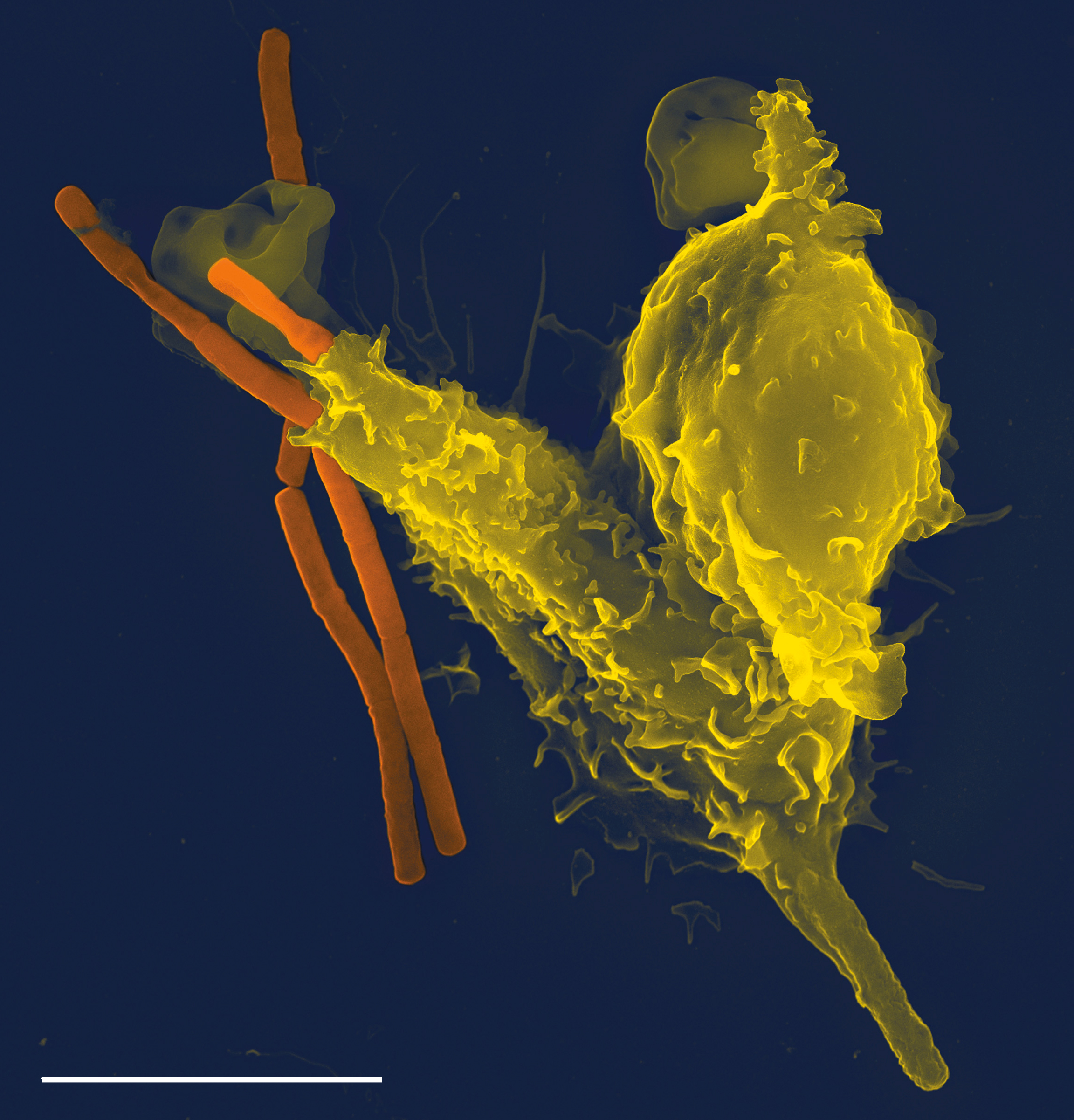
Immune System
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic worms, and also objects such as wood splinters, distinguishing them from the organism's own healthy biological tissue, tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use humoral immunity, molecules and cell-mediated immunity, cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against bacteriophage, viral infections. Other basic immune mechanisms evolved in ancien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biodegradation
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradation occurs under a specific set of circumstances. The process of biodegradation is threefold: first an object undergoes biodeterioration, which is the mechanical weakening of its structure; then follows biofragmentation, which is the breakdown of materials by microorganisms; and finally assimilation, which is the incorporation of the old material into new cells. In practice, almost all chemical compounds and materials are subject to biodegradation, the key element being time. Things like vegetables may degrade within days, while glass and some plastics take many millennia to decompose. A standard for biodegradability used by the European Union is that greater than 90% of the original material must be converted into , water and minerals b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon–fluorine Bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F single bond), and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. For this reason, fluoroalkanes like tetrafluoromethane (carbon tetrafluoride) are some of the most unreactive organic compounds. Electronegativity and bond strength The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine bond a significant polarity or dipole moment. The electron density is concentrated around the fluorine, leaving the carbon relatively electron poor. This introduces ionic character to the bond through partial charges (Cδ+—Fδ−). The partial charges on the fluorine and carbon ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |