|
Organogermanium Compound
Organogermanium chemistry is the science of chemical species containing one or more Carbon, C–Germanium, Ge bonds. Germanium shares Carbon group, group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of Organosilicon chemistry, organosilicon and Organotin chemistry, organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and Organoboron chemistry, organoboron analogues in some cross-coupling reactions. In general, organogermanium chemistry is much less well-developed than the other group-14 congeners, mainly because germanium is expensive. Synthesis The great majority of organogermanium compounds are tetrahedral with the formula GeR4−nXn where X = H, Cl, etc. Ge-C bonds are air-stable, although Ge-H bonds can undergo air-oxidation. The first organogermanium compound, tetraethylgermane, synthesized by Winkler in 1887, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
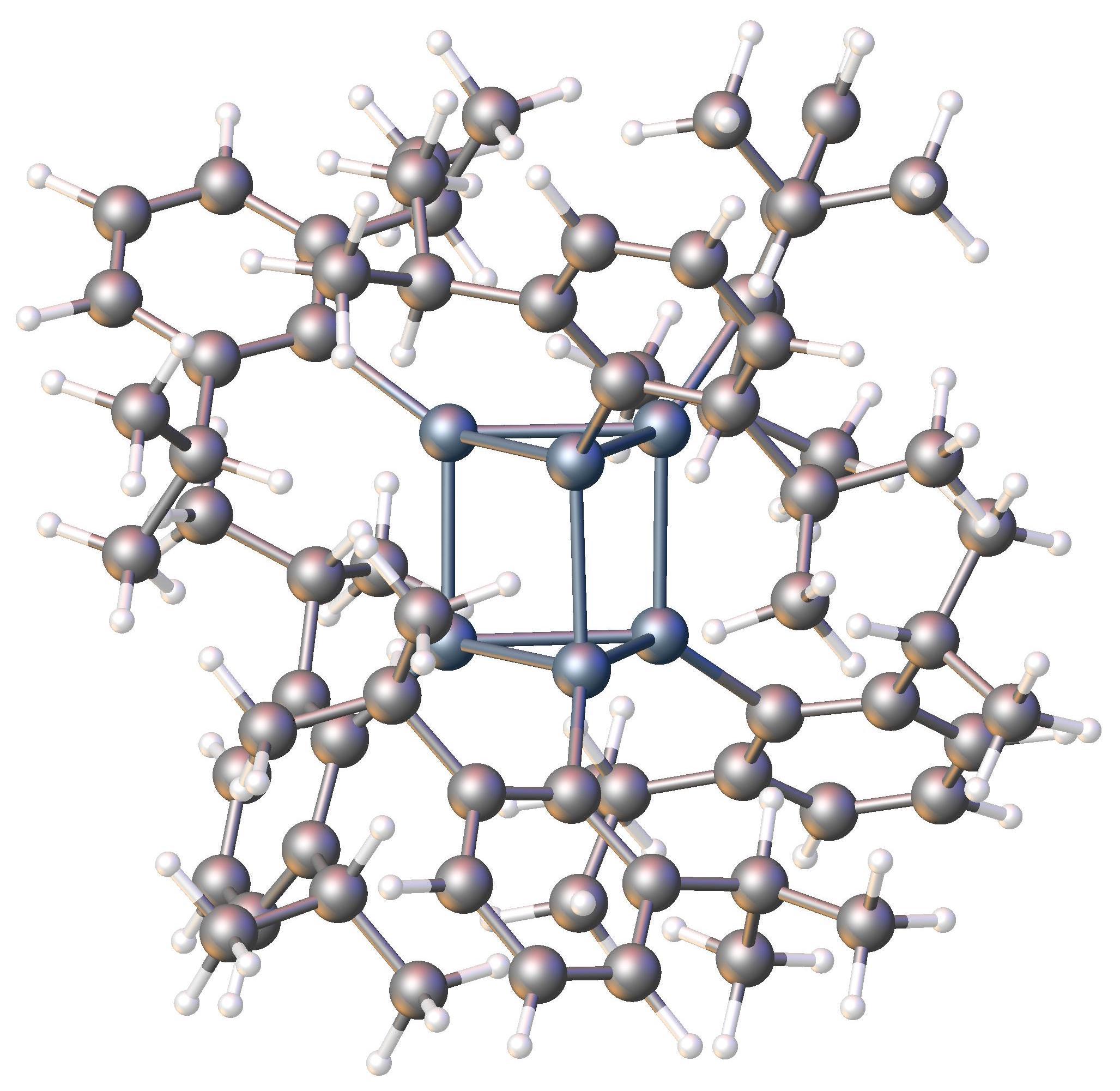
CSD CIF WAVFUU
CSD may refer to: Finance * Central securities depository * Confederate States Dollar * Serbian dinar, by previous ISO 4217 code Organizations Education * California School for the Deaf (other), several institutions * Canyons School District, in Utah, US * Cheltenham School District, in Pennsylvania, US * Christina School District, in Delaware, US * Cleveland School District, in Mississippi, US * Cordova School District, in Alaska, US Other organizations * Canteen Stores Department (India), a chain of stores operated by the Indian Ministry of Defence at military bases * CSD Pakistan (Canteen Stores Department), a chain of stores operated by the Pakistani Ministry of Defence * Chartered Society of Designers, a British learned society for various kinds of design work * Commission on Sustainable Development (1992–2013), a former UN agency * Communication Service for the Deaf, an American non-profit company providing ASL services * Congress of Democratic Tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels are present in the same crystal, they form a semiconductor junction. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called " metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits. Semiconductor devices can display a range of different useful properties, such as passing current more easil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epitaxy
Epitaxy (prefix ''epi-'' means "on top of”) is a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epitaxial film or epitaxial layer. The relative orientation(s) of the epitaxial layer to the seed layer is defined in terms of the orientation of the crystal lattice of each material. For most epitaxial growths, the new layer is usually crystalline and each crystallographic domain of the overlayer must have a well-defined orientation relative to the substrate crystal structure. Epitaxy can involve single-crystal structures, although grain-to-grain epitaxy has been observed in granular films. For most technological applications, single-domain epitaxy, which is the growth of an overlayer crystal with one well-defined orientation with respect to the substrate crystal, is preferred. Epitaxy can also play an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalorganic
Metal-organic compounds (jargon: metalorganics, metallo-organics) are a class of chemical compounds that contain metals and organic ligands, but lacking direct metal-carbon bonds. Metal β-diketonates, metal alkoxides, metal dialkylamides, transition metal carboxylate complexes, metal acetylacetonates, and metal phosphine complexes are representative members of this class. Some of metal-organic compounds confer solubility in organic solvents or volatility. Compounds with these properties find applications in materials science for metal organic vapor deposition (MOCVD) or sol-gel processing. Precise definitions of metal-organic compound may vary, however the term may describe: *Organometallic chemistry * Metal coordination complex A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' .. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOVPE
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as light-emitting diodes, its most widespread application. It was first demonstrated in 1967 at North American Aviation (later Rockwell International) Autonetics Division in Anaheim CA by Harold M. Manasevit. Basic principl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutylgermane
Isobutylgermane (IBGe, Chemical formula: (CH3)2CHCH2GeH3, is an organogermanium compound. It is a colourless, volatile liquid that is used in MOVPE (Metalorganic Vapor Phase Epitaxy) as an alternative to germane. IBGe is used in the deposition of Ge films and Ge-containing thin semiconductor films such as SiGe in strained silicon application, and GeSbTe in NAND Flash applications. Properties IBGe is a non- pyrophoric liquid source for chemical vapor deposition ( CVD) and atomic layer deposition (ALD) of semiconductors. It possesses very high vapor pressure and is considerably less hazardous than germane gas. IBGe also offers lower decomposition temperature (the onset of decomposition at ca. 325-350 °C).,Safer alterna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germylene
Germylenes are a class of germanium(II) compounds with the general formula :GeR2. They are heavier Carbene analog, carbene analogs. However, unlike Carbene, carbenes, whose ground state can be either Singlet state, singlet or Triplet state, triplet depending on the Substituent, substituents, germylenes have exclusively a singlet ground state. Unprotected carbene analogs, including germylenes, has a Dimer (chemistry), dimerization nature. Free germylenes can be isolated under the stabilization of Steric effects#Steric hindrance, steric hindrance or Electron donor, electron donation. The synthesis of first stable free dialkyl germylene was reported by Jutzi, et al in 1991. Structures and bonding Bonding situation for germylene is distinctively different from that for its light analog carbene. The carbon atom from carbene is sp2 Orbital hybridisation, hybridized. Although germylenes still have some sp2 Orbital hybridisation, hybridization character, the larger energy gap between Atomi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organogermanium Compounds
Organogermanium chemistry is the science of chemical species containing one or more C– Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions. In general, organogermanium chemistry is much less well-developed than the other group-14 congeners, mainly because germanium is expensive. Synthesis The great majority of organogermanium compounds are tetrahedral with the formula GeR4−nXn where X = H, Cl, etc. Ge-C bonds are air-stable, although Ge-H bonds can undergo air-oxidation. The first organogermanium compound, tetraethylgermane, synthesized by Winkler in 1887, by the reaction of germanium tetrachloride with diethylzinc. More commonly, these Ge(IV) compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond Rule
In chemistry, the double bond rule states that elements with a principal quantum number (''n'') greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form multiple bonds (e.g. double bonds and triple bonds). Double bonds for these heavier elements, when they exist, are often weak due to poor orbital overlap between the ''n''>2 orbitals of the two atoms. Although such compounds are not intrinsically unstable, they instead tend to dimerize or even polymerize In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form .... Moreover, the multiple bonds of the elements with ''n''=2 are much stronger than usual, because lone pair repulsion weakens their sigma bonding but not their pi bonding. An example is the rapid polymerization that occurs upon condensation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon chemistry and silicate mineralogy. If a silanol contains one or more organic residues, it is an organosilanol. Preparation From alkoxysilanes The first isolated example of a silanol was , reported in 1871 by Albert Ladenburg. He prepared the “silicol” by hydrolysis of (Et = ). From silyl halides and related compounds Silanols are generally synthesized by hydrolysis of halosilanes, alkoxysilanes, or aminosilanes. Chlorosilanes are the most common reactants: :R3Si–Cl + H2O → R3Si–OH + HCl The hydrolysis of fluorosilanes requires more forcing reagents, i.e. alkali. The alkoxysilanes ( silyl ethers) of the type are slow to hydrolyze. Compared to the silyl ethers, silyl acetates are faster to hydrolyze, with th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |