|
Organobromine Compound
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application of synthetic organobromine compounds is the use of polybrominated diphenyl ethers as fire-retardants, and in fact fire-retardant manufacture is currently the major industrial use of the element bromine. A variety of minor organobromine compounds are found in nature, but none are biosynthesized or required by mammals. Organobromine compounds have fallen under increased scrutiny for their environmental impact. General properties Most organobromine compounds, like most organohalide compounds, are relatively nonpolar. Bromine is more electronegative than carbon (2.9 vs 2.5). Consequently, the carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating agents. Carbon–haloge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Wiley & Sons
John Wiley & Sons, Inc., commonly known as Wiley (), is an American Multinational corporation, multinational Publishing, publishing company that focuses on academic publishing and instructional materials. The company was founded in 1807 and produces books, Academic journal, journals, and encyclopedias, in print and electronically, as well as online products and services, training materials, and educational materials for undergraduate, graduate, and continuing education students. History The company was established in 1807 when Charles Wiley opened a print shop in Manhattan. The company was the publisher of 19th century American literary figures like James Fenimore Cooper, Washington Irving, Herman Melville, and Edgar Allan Poe, as well as of legal, religious, and other non-fiction titles. The firm took its current name in 1865. Wiley later shifted its focus to scientific, Technology, technical, and engineering subject areas, abandoning its literary interests. Wiley's son Joh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoiodine Compound
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt. Structure, bonding, general properties Almost all organoiodine compounds feature iodide connected to one carbon center. These are usually classified as derivatives of I−. Some organoiodine compounds feature iodine in higher oxidation states. The C–I bond is the weakest of the carbon–halogen bonds. These bond strengths correlate with the electronegativity of the halogen, decreasing in the order F > Cl > Br > I. This periodic order also follows the atomic radius of halogens and the length of the carbon-halogen bond. For example, in the molecules represented by CH3X, where X ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
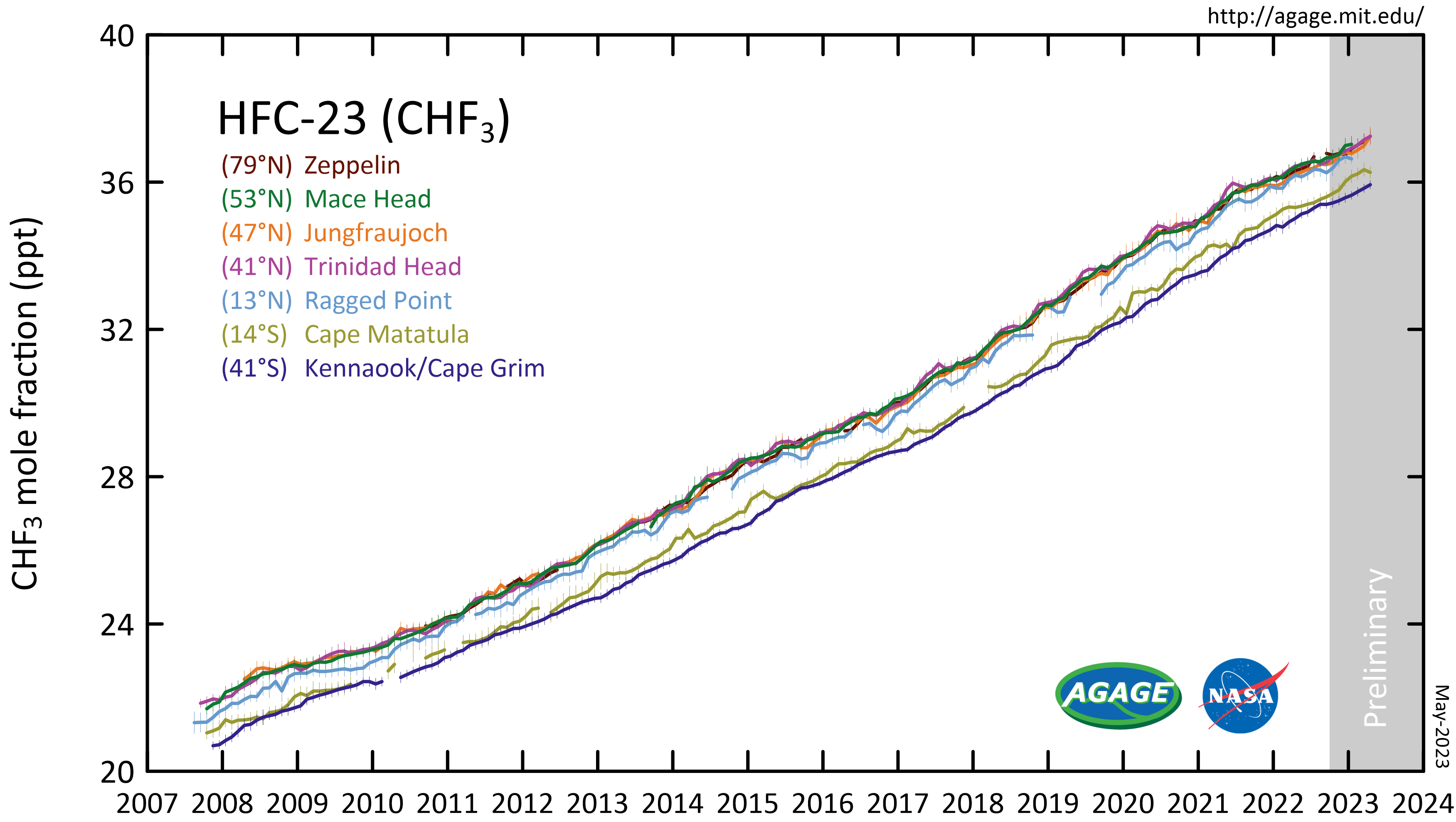
Bromotrifluoromethane
Bromotrifluoromethane, commonly referred to by the code numbers Halon 1301, R13B1, Halon 13B1 or BTM, is an organic halide with the chemical formula carbon, Cbromine, Brfluorine, F3. It is used for gaseous fire suppression as a far less toxic alternative to bromochloromethane. Table of physical properties Synthesis Bromotrifluoromethane is commercially synthesized in a two-step process from chloroform. Chloroform is fluorinated with hydrogen fluoride. CHCl3 + 3 HF → CHF3 + 3 HCl The resulting Fluoroform is then reacted with elemental bromine. CHF3 + Br2 → CF3Br + HBr Uses Halon 1301 was developed in a joint venture between the U.S. Army and Purdue University in the late 1940's, and became a DuPont product in 1954. It was introduced as an effective gaseous fire suppression fixed systems agent in the 1960s, and was used around valuable materials, such as aircraft, Mainframe computer, mainframe computers, and telecommunication telephone exchange, switching centers, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroform
Fluoroform, or trifluoromethane, is the chemical compound with the formula . It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas. Synthesis About 20 million kg per year are produced industrially as both a by-product of and precursor to the manufacture of Teflon. It is produced by reaction of chloroform with HF: : It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid. Historical Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of mercury fluoride and calcium fluoride. The exchange reaction works with iodoform and bromoform, and the exchange of the first two haloge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Tribromide
Phosphorus tribromide is a colourless liquid with the formula P Br3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides. Preparation PBr3 is prepared by treating red phosphorus with bromine. An excess of phosphorus is used in order to prevent formation of PBr5: :P4 + 6 Br2 → 4 PBr3 Because the reaction is highly exothermic, it is often conducted in the presence of a diluent such as PBr3. Phosphorus tribromide is also generated in situ from red phosphorus and bromine. Reactions Phosphorus tribromide, like PCl3 and PF3, has both properties of a Lewis base and a Lewis acid. For example, with a Lewis acid such as boron tribromide it forms stable 1 :1 adducts such as Br3B · PBr3. At the same time PBr3 can react as an electrophile or Lewis acid in many of its reactions, for example with amines. An important reaction of PBr3 is with alcohols, where it replaces an OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoacetic Acid
Bromoacetic acid is a chemical compound with the formula . This colorless solid is a relatively strong alkylating agent. Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example, in pharmaceutical chemistry. The compound is prepared by bromination of acetic acid, such as by a Hell–Volhard–Zelinsky reaction or using other reagent In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...s.. : References External links The microwave spectrum of bromoacetic acid {{DEFAULTSORT:Bromoacetic Acid Alkylating agents Acetic acids Organobromides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisphenol-A
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is Solubility, soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes. BPA's largest single application is as a co-monomer in the production of polycarbonates, which accounts for 65–70% of all BPA production. The manufacturing of epoxy resins and vinyl ester resins account for 25–30% of BPA use. The remaining 5% is used as a major component of several high-performance plastics, and as a minor additive in polyvinyl chloride (PVC), polyurethane, thermal paper, and several other materials. It is not a plasticizer, although it is often wrongly labelled as such. The health effects of BPA have been the subject of prolonged public and scientific debate. BPA is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabromobisphenol-A
Tetrabromobisphenol A (TBBPA) is a brominated flame retardant. The compound is a white solid (not colorless), although commercial samples appear yellow. It is one of the most common flame retardants. Production and use TBBPA is produced by the reaction of bromine with bisphenol A. Most commercial TBBPA products consist of a mixture that differ in the degree of bromination with the formula C15H16−xBrxO2 where x = 1 to 4. Its fire-retarding properties correlate with its bromine content. The annual consumption in Europe has been estimated as 6200 tons in 2004. TBBPA is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant polycarbonates by replacing some bisphenol A. A lower grade of TBBPA is used to prepare epoxy resins, used in printed circuit boards. Toxicity A study was published by the European Food Safety Authority (EFSA) in December 2011 on the exposure of TBBPA and its derivativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The Aromatic sulfonation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at . Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached. Hydrogen bromide, and its aqueous solution, hydrobromic acid, are commonly used reagents in the preparation of bromide compounds. Reactions Organic chemistry Hydrogen bromide and hydrobromic acid are important reagents in the production of organobromine compounds.Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heineman: Oxford, Great Britain; 1997; pp. 809–812.Vollhardt, K. P. C.; Neil E. Schore, Schore, N. E. Organic Chemistry: Structure and Function; 4th Ed.; W. H. Freeman and Company: New York, N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicinal (chemistry)
In chemistry the descriptor vicinal (from Latin ''vicinus'' = neighbor), abbreviated ''vic'', is a descriptor that identifies two functional groups as bonded to two adjacent carbon atoms (i.e., in a 1,2-relationship). It may arise from vicinal difunctionalization. Relation of atoms in a molecule For example, the molecule 2,3-dibromobutane carries two vicinal bromine Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ... atoms and 1,3-dibromobutane does not. Mostly, the use of the term vicinal is restricted to two identical functional groups. Likewise in a ''gem-''dibromide the prefix ''gem'', an abbreviation of '' geminal'', signals that both bromine atoms are bonded to the same carbon atom (i.e., in a 1,1-relationship). For example, 1,1-dibromobutane is geminal. While comparativel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
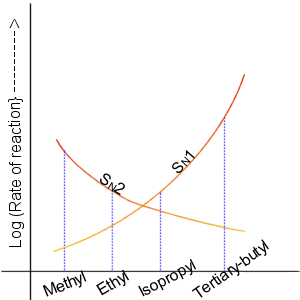
Nucleophilic Substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |