|
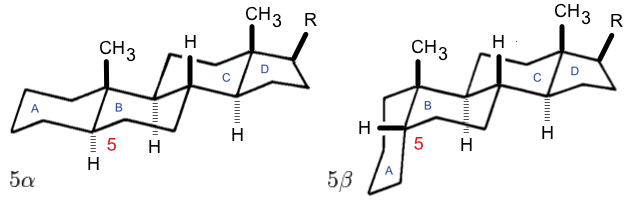
Oleanane
Oleanane is a natural triterpenoid. It is commonly found in woody angiosperms and as a result is often used as an indicator of these plants in the fossil record. It is a member of the oleanoid series, which consists of pentacyclic triterpenoids (such as beta-amyrin and taraxerol) where all rings are six-membered. Structure Oleanane is a pentacyclic triterpenoid, a class of molecules made up of six connected isoprene units. The naming of both the ring structures and individual carbon atoms in oleanane is the same as in steroid A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes t ...s. As such, it consists of a A, B, C, D, and E ring, all of which are six-membered rings. The structure of oleanane contains a number of different methyl groups, that vary in orientation between different o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taraxerol
Taraxerol is a naturally-occurring pentacyclic Triterpene, triterpenoid. It exists in various higher plants, including ''Taraxacum officinale'' (Asteraceae), ''Alnus glutinosa'' (Betulaceae), Litsea, ''Litsea dealbata'' (Lauraceae), Skimmia, ''Skimmia spp.'' (Rutaceae), Dorstenia, ''Dorstenia spp.'' (Moraceae), Maytenus, ''Maytenus spp.'' (Celastraceae), and ''Alchornea latifolia'' (Euphorbiaceae, Euphobiaceae). Taraxerol was named "alnulin" when it was first isolated in 1923 from the bark of the grey alder (''Alnus incana'' L.) by Zellner and Röglsperger. It also had the name "skimmiol" when Takeda and Yosiki isolated it from ''Skimmia'' (Rutaceae). A large number of medicinal plants are known to have this compound in their leaves, roots or seed oil. Chemistry Structure Taraxerol is an oleanan-3-ol with an alpha-Methyl group, methyl substituent at position 13, a missing methyl group at position 14, and a double bond between 14 and 15. The dominant biological Stereoisomerism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpenoid
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' often are used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyrin
The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton), and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee. A study demonstrated that α,β-amyrin exhibits long-lasting antinociceptive and anti-inflammatory properties in 2 models of persistent nociception via activation of the cannabinoid receptors CB1 and CB2 and by inhibiting the production of cytokines and expression of NF-κB, CREB, and cyclooxygenase 2. References {{reflist Secondary alcohols Triterpenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hopane
Hopane is a natural chemical compound classified as a triterpene. It forms the central core of a variety of other chemical compounds which are collectively known as hopanoids. The first compound of the hopane family to be isolated and characterised was hydroxyhopanone, found in dammar resin. The name derives from '' Hopea'', a tree genus from which dammar is obtained. See also * Bacteriohopanepolyol References Triterpenes Hydrocarbons Cyclopentanes Isopropyl compounds {{alkanederivative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oleanolic Acid
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins. Natural occurrence Oleanolic acid can be found in olive oil, '' Phytolacca americana'' (American pokeweed), and '' Syzygium'' spp, garlic, etc. It was first studied and isolated from several plants, including '' Olea europaea'' (leaves, fruit), '' Rosa woodsii'' (leaves), '' Prosopis glandulosa'' (leaves and twigs), '' Phoradendron juniperinum'' (whole plant), '' Syzygium claviflorum'' (leaves), '' Hyptis capitata'' (whole plant), '' Mirabilis jalapa'') and '' Ternstroemia gymnanthera'' (aerial part). Other ''Syzygium'' species including java apple ('' Syzygium samarangense'') and rose apples contain it, as does '' Ocimum tenuiflorum'' (holy basil). Biosynthesis of oleanolic acids Oleanolic acid biosynthesis starts with mevalonate to create squalene. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Nonaromatic Hydrocarbons
Polycyclic may refer to: * Polycyclic compound, a cyclic compound with more than one hydrocarbon loop or ring structures, including: ** Polycyclic musks ** Polycyclic aromatic hydrocarbon A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incine ... *** Chlorinated polycyclic aromatic hydrocarbon *** Contorted polycyclic aromatic hydrocarbon * Polycyclic group, in mathematics, a solvable group that satisfies the maximal condition on subgroups * Polycyclic spawning, when an animal reproduces multiple times during its lifespan {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |