|
Non-nucleophilic Base
As the name suggests, a non-nucleophilic base is a sterically hindered organic base that is a poor nucleophile. Normal bases are also nucleophiles, but often chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach to the basic center but alkylation and complexation is inhibited. Non-nucleophilic bases A variety of amines and nitrogen heterocycles are useful bases of moderate strength (pKa of conjugate acid around 10-13) * ''N'',''N''-Diisopropylethylamine (DIPEA, also called Hünig's Base), pKa = 10.75 *1,8-Diazabicycloundec-7-ene (DBU) - useful for E2 elimination reactions, pKa = 13.5 * 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN) - comparable to DBU * 2,6-Di-tert-butylpyridine, a weak non-nucleophilic base pKa = 3.58 * Phosphazene bases, such as ''t''-Bu-P4''Activation in anionic polymerization: Why phosphazene bases are very exciting promoters'' S. Boileau, N. Illy Prog. Polym. Sci., 20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, conformation) and chemical reaction, reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bis(trimethylsilyl)amide
Potassium is a chemical element; it has symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a common constituent of granites and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β- diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. The reaction has often been displaced by diketene-based chemistry, which affords acetoacetic esters. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene"/"alkene" and the "-ol". Many kinds of enols are known. Keto–enol tautomerism refers to a chemical equilibrium between a "keto" form (a carbonyl, named for the common ketone case) and an enol. The interconversion of the two forms involves the transfer of an alpha hydrogen atom and the reorganisation of bonding electrons. The keto and enol forms are tautomers of each other. Enolization Organic esters, ketones, and aldehydes with an α-hydrogen ( bond adjacent to the carbonyl group) often form enols. The reaction involves migration of a proton () from carbon to oxygen: : In the case of ketones, the conversion is called a keto-enol tautomerism, although this name is often more generally applied to all such tautomerizations. Usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu/hjakubowski/classes/ch331/protstructure/PS_2A3_AA_Charges.html, accessed 12/2/2020 The species formed is the conjugate base of that acid. The complementary process, when a proton is added (transferred) to a Brønsted–Lowry base, is protonation (or hydronation). The species formed is the conjugate acid of that base. A species that can either accept or donate a proton is referred to as amphiprotic. An example is the H2O (water) molecule, which can gain a proton to form the hydronium ion, H3O+, or lose a proton, leaving the hydroxide ion, OH−. The relative ability of a molecule to give up a proton is measured by its p''K''a value. A low p''K''a value indicates that the compound is acidic and will easily give up its proton to a base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
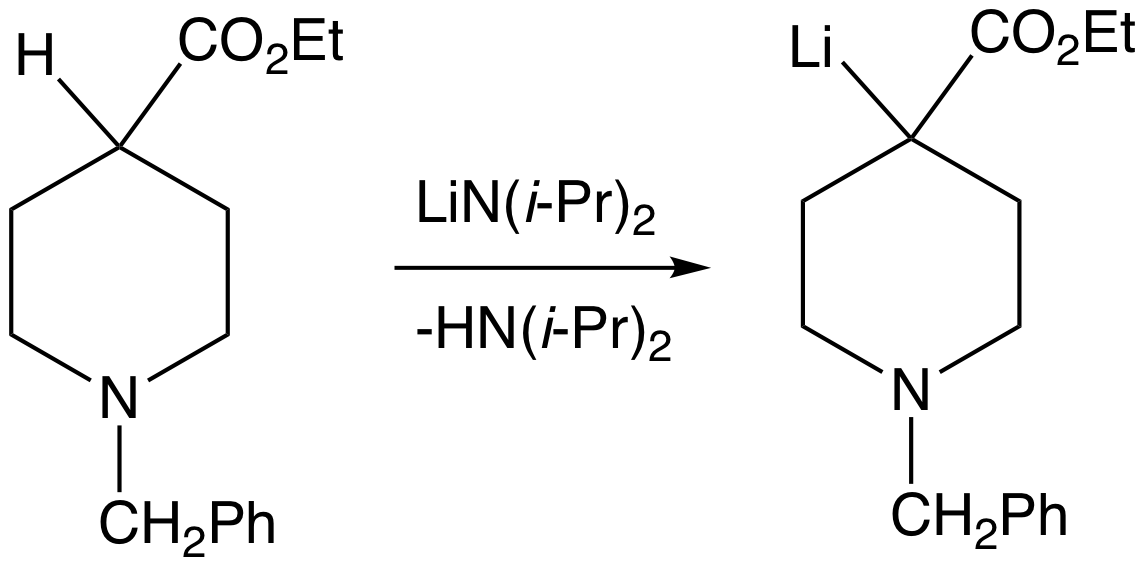
Lithium Diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group. Preparation and structure LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of tetrahydrofuran and diisopropylamine with ''n''-butyllithium. When dissociated, the diisopropylamide anion can become protonated to form diisopropylamine. Diisopropylamine has a p''K''a value of 36. Therefore, its conjugate base is suitable for the deprotonation of compounds with greater acidity, importantly, such weakly acidic compounds (carbon acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Tert-butoxide
Potassium ''tert''-butoxide (or potassium ''t''-butoxide) is a chemical compound with the formula CH3)3COKsub>''n'' (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid is 17 in H2O), which is useful in organic synthesis. The compound is often depicted as a salt, and it often behaves as such, but its ionization depends on the solvent. Preparation Potassium ''t''-butoxide is commercially available as a solution and as a solid, but it is often generated ''in situ'' for laboratory use because samples are so moisture- sensitive and older samples are often of low purity. It is prepared by the reaction of dry ''tert''-butyl alcohol with potassium metal. The solid is obtained by evaporating these solutions followed by heating the solid. The solid can be purified by sublimation. Structure It crystallizes as a tetrameric cubane-type cluster. It crystallises from tetrahydrofuran/pentane at −20 °C as BuOK·tBuOHsub>∞, which consists of straig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Tert-butoxide
Sodium ''tert''-butoxide (or sodium ''t''-butoxide) is a chemical compound with the formula (CH3)3CONa (abbr. NaOtBu). It is a strong, non-nucleophilic base. It is flammable and moisture sensitive. It is sometimes written in the chemical literature as sodium ''t''-butoxide. It is similar in reactivity to the more common potassium ''tert''-butoxide. The compound can be produced by treating ''tert''-butyl alcohol with sodium hydride. Reactions One application for sodium ''tert''-butoxide is as a non-nucleophilic base. It has been widely used in the Buchwald–Hartwig amination, as in this typical example: Sodium tert-butoxide is used to prepare tert-butoxide complexes. For example hexa(tert-butoxy)ditungsten(III) is thus obtained by the salt metathesis reaction from a ditungsten heptachloride: :NaW2Cl7(THF)5 + 6 NaOBu-t → W2(OBu-t)6 + 7 NaCl + 5 THF Structure Sodium ''tert''-butoxide forms clusters in the solid state, both hexamers and nonamers. Related compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold commercially as a slurry (~35%) in mineral oil or sometimes paraffin wax to facilitate dispensing. Preparation Potassium hydride is produced by direct combination of the metal and hydrogen at temperatures between 200 and 350 °C: : This reaction was discovered by Humphry Davy soon after his 1807 discovery of potassium, when he noted that the metal would vaporize in a current of hydrogen when heated just below its boiling point.Humphry Davy (1808), ''The Bakerian Lecture on some new phenomena of chemical changes produced by electricity, particularly the decomposition of fixed alkalies, and the exhibition of the new substances which constitute their bases; and on the general nature of alkaline bodies.'' Philosophical Transactions of the Ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, silane, germane, ammonia, and methane. It is an ionic material that is insoluble in all solvents (other than molten sodium metal), consistent with the fact that H− ions do not exist in solution. Basic properties and structure NaH is colorless, although samples generally appear grey. NaH is around 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H− centers in an octahedral geometry. The ionic radii of H− (146 pm in NaH) and F− (133 pm) are comparable, as judged by the Na−H and Na−F distances. "Inverse sodium hydride" (hydrogen sodide) A very unusual situation occurs in a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Tetramethylpiperidide
Lithium tetramethylpiperidide (often abbreviated LiTMP or LTMP) is a chemical compound with the molecular formula . It is used as a non-nucleophilic base, being comparable to LiHMDS in terms of steric hindrance. Synthesis It is synthesised by the deprotonation of 2,2,6,6-tetramethylpiperidine with ''n''-butyllithium at −78 °C. Recent reports show that this reaction can also be performed 0 °C. The compound is stable in a THF/ethylbenzene solvent mixture and is commercially available as such. Structure Like many lithium reagents it has a tendency to aggregate, forming a tetramer in the solid state. See also *Lithium diisopropylamide *Lithium amide Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia: : Lithium amide decomposes into ... References {{Lithium compounds Lithium compounds Non-nucleophilic bases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |