|
Neutron Poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable effect. However, neutron-absorbing materials, also called poisons, are intentionally inserted into some types of reactors in order to lower the high reactivity of their initial fresh fuel load. Some of these poisons deplete as they absorb neutrons during reactor operation, while others remain relatively constant. The capture of neutrons by short half-life fission products is known as reactor poisoning; neutron capture by long-lived or stable fission products is called reactor slagging. Transient fission product poisons Some of the fission products generated during nuclear reactions have a high neutron absorption capacity, such as xenon-135 (microscopic cross-section σ = 2,000,000 barns (b); up to 3 million barns in reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons production and Research reactor, research. Fissile material, Fissile nuclei (primarily uranium-235 or plutonium-239) absorb single neutron, neutrons and split, releasing energy and multiple neutrons, which can induce further fission. Reactors stabilize this, regulating Neutron absorber, neutron absorbers and neutron moderator, moderators in the core. Fuel efficiency is exceptionally high; Enriched uranium#Low-enriched uranium (LEU), low-enriched uranium is 120,000 times more energy dense than coal. Heat from nuclear fission is passed to a working fluid Nuclear reactor#By coolant, coolant. In commercial reactors, this drives Turbine, turbines and electrical generator shafts. Some reactors are used for district heating, and isotopes, isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spent Nuclear Fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started. Nuclear fuel rods become progressively more radioactive (and less thermally useful) due to neutron activation as they are fissioned, or "burnt", in the reactor. A fresh rod of low enriched uranium pellets (which can be safely handled with gloved hands) will become a highly lethal gamma emitter after 1–2 years of core irradiation, unsafe to approach unless under many feet of water shielding. This makes their invariable accumulation and safe temporary storage in spent fuel pools a prime source of high level radioactive waste and a major ongoing issue for future permanent disposal. Nature of spent fuel Nanomaterial pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled Electrostatics, electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of Mass number, masses greater than 56 Iron peak, cannot be formed by exothermic thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope gold-198, 198A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Cooled Fast Reactor
The lead-cooled fast reactor is a nuclear reactor design that uses molten lead or lead-bismuth eutectic as its coolant. These materials can be used as the primary coolant because they have low neutron absorption and relatively low melting points. Neutrons are slowed less by interaction with these heavy nuclei (thus not being neutron moderators) so these reactors operate with fast neutrons. The concept is generally similar to sodium-cooled fast reactors, and most liquid-metal fast reactors have used sodium instead of lead. Few lead-cooled reactors have been constructed, except for the Soviet submarine K-27 and the seven Soviet Alfa-class submarines (though these were beryllium-moderated intermediate energy reactors rather than fast reactors). Some proposed new nuclear reactor designs are lead-cooled. Fuel designs being explored for this reactor scheme include fertile uranium as a metal, metal oxide or metal nitride. The lead-cooled reactor design has been proposed as a gener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead-bismuth Eutectic
Lead-bismuth eutectic or LBE is a eutectic alloy of lead (44.5 at%) and bismuth (55.5 at%) used as a coolant in some nuclear reactors, and is a proposed coolant for the lead-cooled fast reactor, part of the Generation IV reactor initiative. It has a melting point of 123.5 °C/254.3 °F (pure lead melts at 327 °C/621 °F, pure bismuth at 271 °C/520 °F) and a boiling point of 1,670 °C/3,038 °F. Lead-bismuth alloys with between 30% and 75% bismuth all have melting points below 200 °C/392 °F. Alloys with between 48% and 63% bismuth have melting points below 150 °C/302 °F. While lead expands slightly on melting and bismuth contracts slightly on melting, LBE has negligible change in volume on melting. History The Soviet Alfa-class submarines used LBE as a coolant for their nuclear reactors throughout the Cold War. OKB Gidropress (the Russian developers of the VVER-type Light-water reactors) has expertise in LBE ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Neutrons
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwell distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The long wavelength of slow neutrons allows for the large cross section. Neutron energy distribution ranges The precise boundaries of neutron energy ranges are not well defined, and differ between sources, but some common names and limits are given in the following table. The following is a detailed classification: Thermal A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or 2.4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwell distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The long wavelength of slow neutrons allows for the large cross section. Neutron energy distribution ranges The precise boundaries of neutron energy ranges are not well defined, and differ between sources, but some common names and limits are given in the following table. The following is a detailed classification: Thermal A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
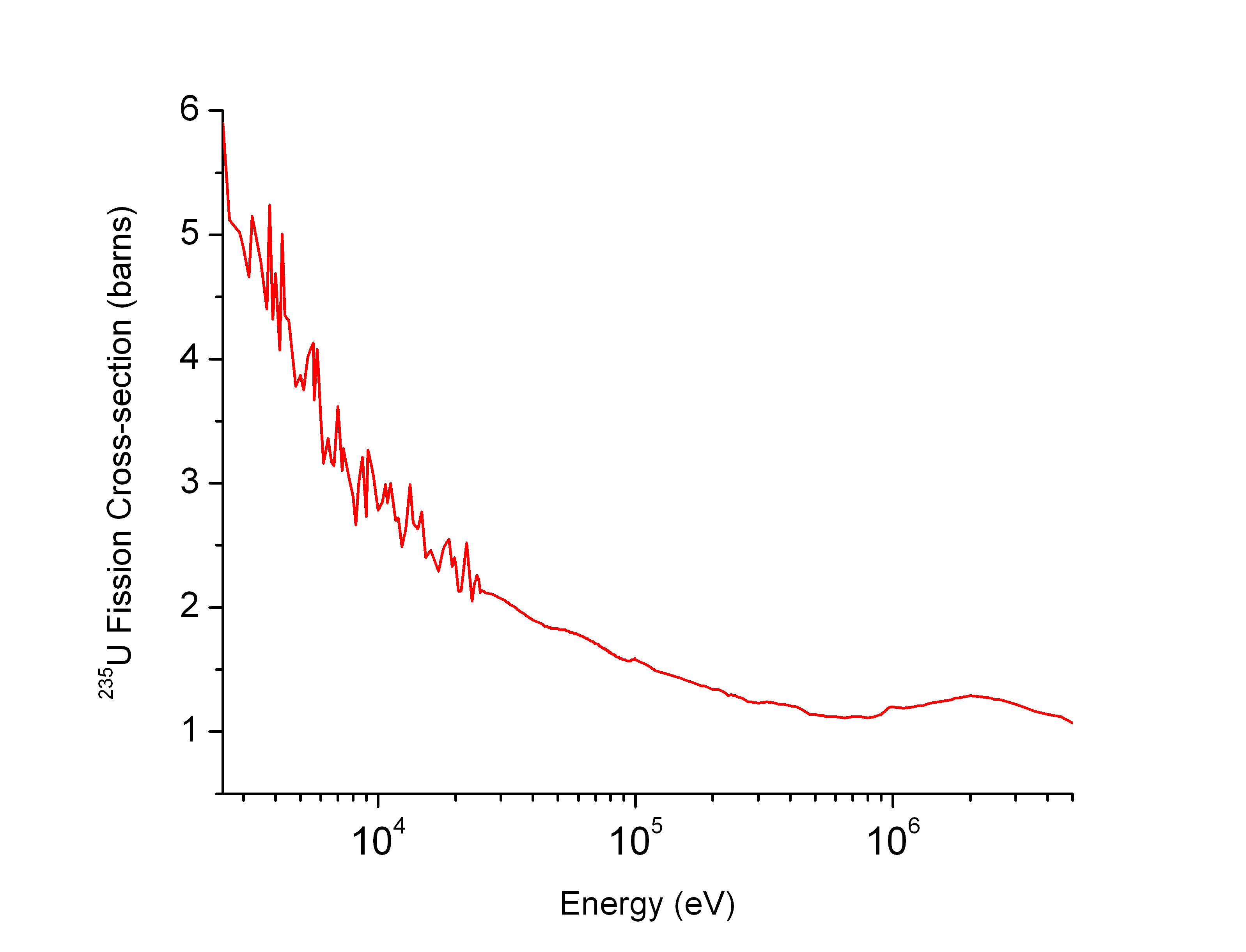
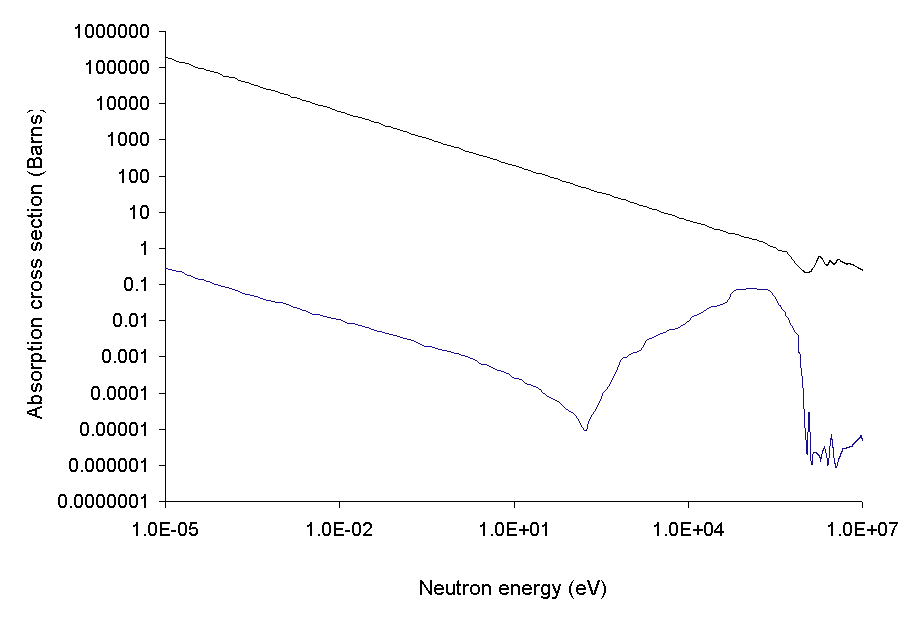
Neutron Cross-section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Absorption
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by exothermic thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and qu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Reactor
A fast-neutron reactor (FNR) or fast-spectrum reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons (carrying energies above 1 MeV, on average), as opposed to slow thermal neutrons used in thermal-neutron reactors. Such a fast reactor needs no neutron moderator, but requires fuel that is comparatively rich in fissile material. The fast spectrum is key to breeder reactors, which convert highly abundant uranium-238 into fissile plutonium-239, without requiring enrichment. It also leads to high burnup: many transuranic isotopes, such as of americium and curium, accumulate in thermal reactor spent fuel; in fast reactors they undergo fast fission, reducing total nuclear waste. As a strong fast-spectrum neutron source, they can also be used to transmute existing nuclear waste into manageable or non-radioactive isotopes. These characteristics also cause fast reactors to be judged a higher n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the ''atomic'' (also known as ''isotopic'') mass of the atom expressed in daltons. Since protons and neutrons are both baryons, the mass number ''A'' is identical with the baryon number ''B'' of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number ''Z'' gives the number of neutrons (''N'') in the nucleus: . The mass number is written either after the element name or as a superscript to the left of an element's symbol. For example, the most common isotope of carbon is carbon-12, or , which has 6 protons and 6 neutrons. The full isotope symbol would also have the atomic number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. A transmutation can be achieved either by nuclear reactions (in which an outside particle reacts with a nucleus) or by radioactive decay, where no outside cause is needed. Natural transmutation by stellar nucleosynthesis in the past created most of the heavier chemical elements in the known existing universe, and continues to take place to this day, creating the vast majority of the most common elements in the universe, including helium, oxygen and carbon. Most stars carry out transmutation through fusion reactions involving hydrogen and helium, while much larger stars are also capable of fusing heavier elements up to iron late in their evolution. Elements heavier than iron, such as gold or lead, are created through elemental transmutations that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |