|
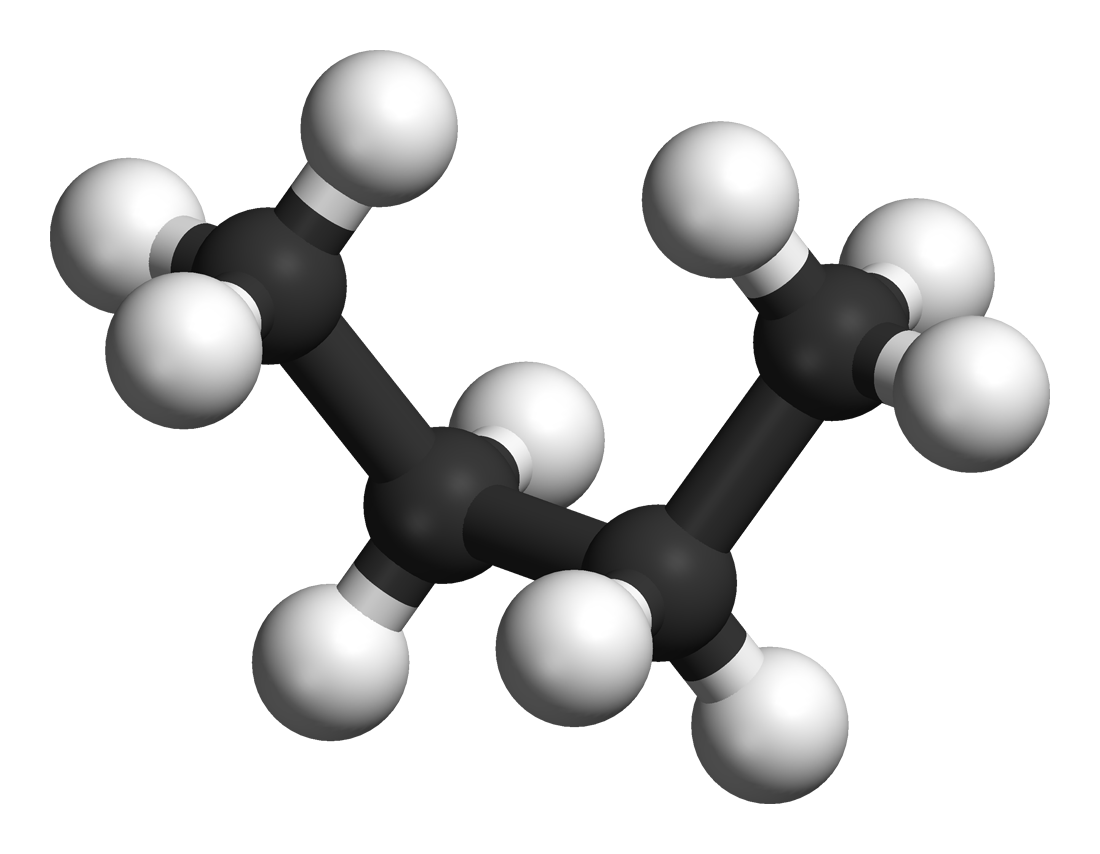
Natta Projection
In chemistry, the Natta projection (named for Italian chemist Giulio Natta) is a way to depict molecules with complete stereochemistry in two dimensions in a skeletal formula. In a hydrocarbon molecule with all carbon atoms making up the backbone in a tetrahedral molecular geometry, the zigzag backbone is in the paper plane (chemical bonds depicted as solid line segments) with the substituents either sticking out of the paper toward the viewer (chemical bonds depicted as solid wedges) or away from the viewer (chemical bonds depicted as dashed wedges). The Natta projection is useful for representing the tacticity of a polymer. See also * Structural formula * Wedge-and-dash notation in skeletal formulas * Haworth projection * Newman projection * Fischer projection In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. Substituents can be a combination of the inductive effect and the mesomeric effect. Such effects are also described as electron-rich and electron withdrawing. Additional steric effects result from the volume occupied by a substituent. The phrases ''most-substituted'' and ''least-substituted'' are frequently used to describe or compare molecules that are products of a chemical reaction. In this terminology, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Projection
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers. Conventions All bonds are depicted as horizontal or vertical lines. The carbon chain is depicted vertically, with carbon atoms sometimes not shown and represented by the center of crossing lines (see figure below). The orientation of the carbon chain is so that the first carbon (C1) is at the top. In an aldose, C1 is the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newman Projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman projection visualizes the Conformational isomerism, conformation of a chemical bond from front to back, with the front atom represented by the intersection of three lines (a dot) and the back atom as a circle. The front atom is called ''proximal'', while the back atom is called ''distal''. This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms. This projection is named after American chemist Melvin Spencer Newman, who introduced it in 1952 as a partial replacement for Fischer projections, which are unable to represent conformations and thus conformers properly.Newman, MS. ''Record. Chem. Progr. (Kresge-Hooker Sci. Lib.) 1952,'' 13'', 111'' This diagram style is an alternative to a sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haworth Projection
In chemistry, a Haworth projection is a common way of writing a structural formula to represent the cyclic structure of monosaccharides with a simple three-dimensional perspective. A Haworth projection approximates the shapes of the actual molecules better for furanoses—which are in reality nearly planar—than for pyranoses that exist in solution in the chair conformation. Organic chemistry and especially biochemistry are the areas of chemistry that use the Haworth projection the most. The Haworth projection was named after the British chemist Sir Norman Haworth. A Haworth projection has the following characteristics: * Carbon is the implicit type of atom. In the example on the right, the atoms numbered from 1 to 6 are all carbon atoms. Carbon 1 is known as the anomeric carbon. * Hydrogen atoms on carbon are implicit. In the example, atoms 1 to 6 have extra hydrogen atoms not depicted. * A thicker line indicates atoms that are closer to the observer. In the example on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skeletal Formula
The skeletal formula, line-angle formula, bond-line formula or shorthand formula of an organic compound is a type of minimalist structural formula representing a molecule's Atom, atoms, structural isomer, bonds and some details of its molecular geometry, geometry. The lines in a skeletal formula represent bonds between carbon atoms, unless labelled with another element. Labels are optional for carbon atoms, and the hydrogen atoms attached to them. An early form of this representation was first developed by organic chemist August Kekulé, while the modern form is closely related to and influenced by the Lewis structure of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures or Lewis–Kekulé structures. Skeletal formulas have become ubiquitous in organic chemistry, partly because they are relatively quick and simple to draw, and also because the Arrow pushing, curved arrow notation used for discussions of reaction mechanisms and Resonance ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections. Several systematic chemical naming formats, as in chemical databases, are used that are equivalent to, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tacticity
Tacticity (from , "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical properties of the polymer. The regularity of the macromolecular structure influences the degree to which it has rigid, crystalline long range order or flexible, amorphous long range disorder. Precise knowledge of tacticity of a polymer also helps understanding at what temperature a polymer melts, how soluble it is in a solvent, as well as its mechanical properties. A tactic macromolecule in the IUPAC definition is a macromolecule in which essentially all the configurational (repeating) units are identical. In a hydrocarbon macromolecule with all carbon atoms making up the backbone in a tetrahedral molecular geometry, the zigzag backbone is in the paper plane with the substituents either sticking out of the paper or retreating into the paper;, this projection is called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Line Segment
In geometry, a line segment is a part of a line (mathematics), straight line that is bounded by two distinct endpoints (its extreme points), and contains every Point (geometry), point on the line that is between its endpoints. It is a special case of an ''arc (geometry), arc'', with zero curvature. The length of a line segment is given by the Euclidean distance between its endpoints. A closed line segment includes both endpoints, while an open line segment excludes both endpoints; a half-open line segment includes exactly one of the endpoints. In geometry, a line segment is often denoted using an overline (vinculum (symbol), vinculum) above the symbols for the two endpoints, such as in . Examples of line segments include the sides of a triangle or square. More generally, when both of the segment's end points are vertices of a polygon or polyhedron, the line segment is either an edge (geometry), edge (of that polygon or polyhedron) if they are adjacent vertices, or a diagonal. Wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Giulio Natta
Giulio Natta (; 26 February 1903 – 2 May 1979) was an Italian chemical engineer and Nobel laureate. He won a Nobel Prize in Chemistry in 1963 with Karl Ziegler for work on high density polymers. He also received a Lomonosov Gold Medal in 1969. Biography Early years Natta was born in Imperia, Italy. He earned his degree in chemical engineering from the Politecnico di Milano university in Milan in 1924. In 1927 he passed the exams for becoming a professor there. From 1929 to 1933, he was also in charge of physical chemistry at the Faculty of Sciences of the University of Milan. In 1933 he became a full professor and the director of the Institute of General Chemistry of Pavia University, where he stayed until 1935. During this time he began using crystallography to elucidate the structures of a wide variety of molecules including phosphine, arsine and others. In that year he was appointed full professor in physical chemistry at the University of Rome. Career From 1936 to 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |