|
NBQX
NBQX (2,3-dioxo-6-nitro-7-sulfamoyl-benzo uinoxaline) is an antagonist of the AMPA receptor. NBQX blocks AMPA receptors in micromolar concentrations (~10–20 μM) and also blocks kainate receptors. In experiments, it is used to counter glutamate excitotoxicity In excitotoxicity, neuron, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamic acid, glutamate become pathologically high, resulting in excessive stimulation of cell surface recept .... NBQX was found to have anticonvulsant activity in rodent seizure models.Yamaguchi, S.; Donevan, S.D.; Rogawski, M.A. (1993). Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. ''Epilepsy Res.'' 15:179–184. As the disodium salt, NBQX is soluble in water at high concentrations (at least up to 100 mM). See also * CNQX * DNQX * Fanapanel (MPQX) * Quinoxalinedione References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoxalinedione
Quinoxalinedione is an organic compound with the formula C6H4(NH)2(CO)2. It is a colorless solid that is soluble in polar organic solvents. Quinoxalinediones are a family of related compounds sharing the same bicyclic core. Various quinoxalinediones are drugs. Synthesis and structure Quinoxalinedione is produced by condensation of dimethyloxalate and o-phenylenediamine: :C2O2(OMe)2 + C6H4(NH2)2 → C6H4(NH)2(CO)2 + 2 MeOH The compound exists in solution and the solid state predominantly as the diamide form. Some reactions of the compound indicate a role for the diol tautomer. Drugs based on quinoxalinediones Quinoxalinediones act as antagonists of the AMPA, kainate, and/or NMDA receptors of the ionotropic glutamate receptor family. Examples include the following: * ACEA-1011 * Becampanel * CNQX * DNQX * Fanapanel (MPQX) * Licostinel (ACEA-1021) * NBQX NBQX (2,3-dioxo-6-nitro-7-sulfamoyl-benzo uinoxaline) is an antagonist of the AMPA receptor. NBQX blocks AMP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNQX
CNQX or cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) is a competitive AMPA/ kainate receptor antagonist. Its chemical formula is C9H4N4O4. CNQX is often used in the retina to block the responses of OFF-bipolar cells for electrophysiology recordings. CNQX is an antagonist of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors (AMPARs). A study of the effects of CNQX on vestibuloocular reflex adaptation was done on goldfish by injecting CNQX into the vestibulo-cerebullum. The injection before adaptation significantly decreased and at the highest doses, completely inhibited the acquisition of adaptive reflex gain increases and decreases during a three-hour training period. Baseline performance was not affected by the CNQX injections. Injections of CNQX at the end of the training period shows a rapid loss of gained vestibuloocular reflex adaptation when the goldfish remained stationary in the dark. Instead of injecting CNQX immediately after t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMPA Receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic receptor, ionotropic glutamate receptor (iGluR) and predominantly sodium ion channel that mediates fast excitatory neurotransmission in the Central nervous system, central nervous system (CNS). Its activation by the neurotransmitter Glutamate (neurotransmitter), glutamate facilitates rapid neuronal communication, essential for various brain functions, including learning and memory. Its name is derived from the ability to be activated by the artificial glutamate analog AMPA. The receptor was initially named the "Quisqualic acid, quisqualate receptor" by Watkins and colleagues after the naturally occurring agonist quisqualic acid, quisqualate. Later, the receptor was designated as the "AMPA receptor" following the development of the selective agonist AMPA by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The ''GRIA2''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves ." '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine Receptor Antagonists
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is Genetic code, encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine disrupts the formation of Alpha helix, alpha-helices in secondary protein structure. Its small side chain causes it to favor random coils instead. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a ''Clostridium tetani'' infection) can cause Spasticity, spastic paralysis due to uninhibited muscle contraction. It is the only chirality (chemistry), achiral proteinogenic amino acid. It can fit into both Hydrophile, hydrophilic and Hydrophobe, hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinones
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words '' lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size. This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on. Synthesis General synthetic methods are used for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms ε - Caprolactum, which upon treatment with excess acid forms Cardiazole, a heart stimulant. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoxalines
A quinoxaline, also called a benzopyrazine, in organic chemistry, is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazoline, phthalazine and cinnoline. It is a colorless oil that melts just above room temperature. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, pharmaceuticals (such as varenicline), and antibiotics such as olaquindox, carbadox, echinomycin, levomycin and actinoleutin. Synthesis They can be formed by condensing ''ortho''-diamines with 1,2-diketones. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene. Substituted derivatives arise when α-ketonic acids, α-chlorketones, α-aldehyde alcohols and α-ketone alcohols are used in place of diketones. Quinoxaline and its analogues may also be formed by reduction of amino acids substituted 1,5-difluoro-2, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
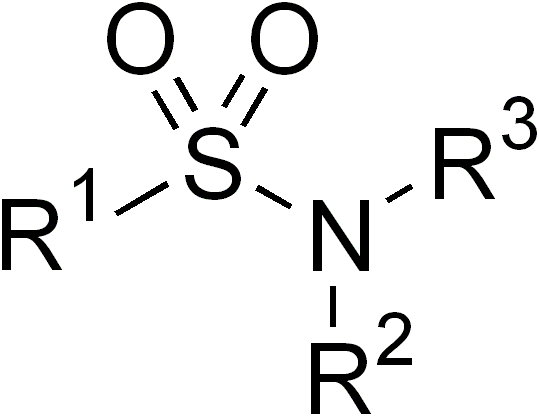
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMPA Receptor Antagonists
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, better known as AMPA, is a compound that is a specific agonist for the AMPA receptor, where it mimics the effects of the neurotransmitter glutamate. There are several types of glutamatergic ion channels in the central nervous system including AMPA, kainic acid and ''N''-methyl-D-aspartic acid (NMDA) channels. In the synapse, these receptors serve very different purposes. AMPA can be used experimentally to distinguish the activity of one receptor from the other in order to understand their differing functions. AMPA generates fast excitatory postsynaptic potentials (EPSP). AMPA activates AMPA receptors that are non-selective cationic channels allowing the passage of Na+ and K+ and therefore have an equilibrium potential near 0 mV. AMPA was first synthesized, along with several other ibotenic acid Ibotenic acid or (''S'')-2-amino-2-(3-hydroxyisoxazol-5-yl)acetic acid, also referred to as ibotenate, is a naturally oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kainate Receptor Antagonists
Kainic acid, or kainate, is an acid that naturally occurs in some seaweed. Kainic acid is a potent neuroexcitatory amino acid agonist that acts by activating receptors for glutamate, the principal excitatory neurotransmitter in the central nervous system. Glutamate is produced by the cell's metabolic processes and there are four major classifications of glutamate receptors: NMDA receptors, AMPA receptors, kainate receptors, and the metabotropic glutamate receptors. Kainic acid is an agonist for kainate receptors, a type of ionotropic glutamate receptor. Kainate receptors likely control a sodium channel that produces excitatory postsynaptic potentials (EPSPs) when glutamate binds. Kainic acid is commonly injected into laboratory animal models to study the effects of Ablative brain surgery, experimental ablation. Kainic acid is a direct agonist of the glutamic kainate receptors and large doses of concentrated solutions produce immediate neuronal death by overstimulating neurons to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |