|
Myosin Light Chain Kinase
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II. General structural features While there are numerous differing domains depending on the cell type, there are several characteristic domains common amongst all MYLK isoforms. MYLK’s contain a catalytic core domain with an ATP binding domain. On either sides of the catalytic core sit calcium ion/calmodulin binding sites. Binding of calcium ion to this domain increases the affinity of MYLK binding to myosin light chain. This myosin binding domain is located at the C-Terminus end of the kinase. On the other side of the kinase at the N-Terminus end, sits the actin-binding domain, which allows MYLK to form interactions with actin filaments, keeping it in place. Isoforms Four different MYLK isoforms exist: * MYLK1 – smooth muscle * MYLK2 – skeletal * MYLK3 – cardiac * MYL ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYLK
Myosin light chain kinase, smooth muscle also known as kinase-related protein (KRP) or telokin is an enzyme that in humans is encoded by the ''MYLK'' gene. Function This gene, a muscle member of the immunoglobulin superfamily, encodes a myosin light-chain kinase, which is a calcium-/ calmodulin-dependent enzyme. This kinase phosphorylates myosin regulatory light chains to facilitate myosin interaction with actin filaments to produce contractile activity. This gene encodes both smooth muscle and nonmuscle isoforms. In addition, using a separate promoter in an intron in the 3' region, it encodes telokin, a small protein identical in sequence to the C-terminus of myosin light chain kinase, that is independently expressed in smooth muscle and functions to stabilize unphosphorylated myosin filaments. A pseudogene is located on the p arm of chromosome 3. Four transcript variants Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in ''Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extra Cellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adherens Junction
Adherens junctions (or zonula adherens, intermediate junction, or "belt desmosome") are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell (zonula adherens) or as spots of attachment to the extracellular matrix (focal adhesion). Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells. A similar cell junction in non-epithelial, non-endothelial cells is the fascia adherens. It is structurally the same, but appears in ribbonlike patterns that do not completely encircle the cells. One example is in cardiomyocytes. Proteins Adherens junctions are composed of the following proteins: * cadherins. The cadherins are a family of transmembrane proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
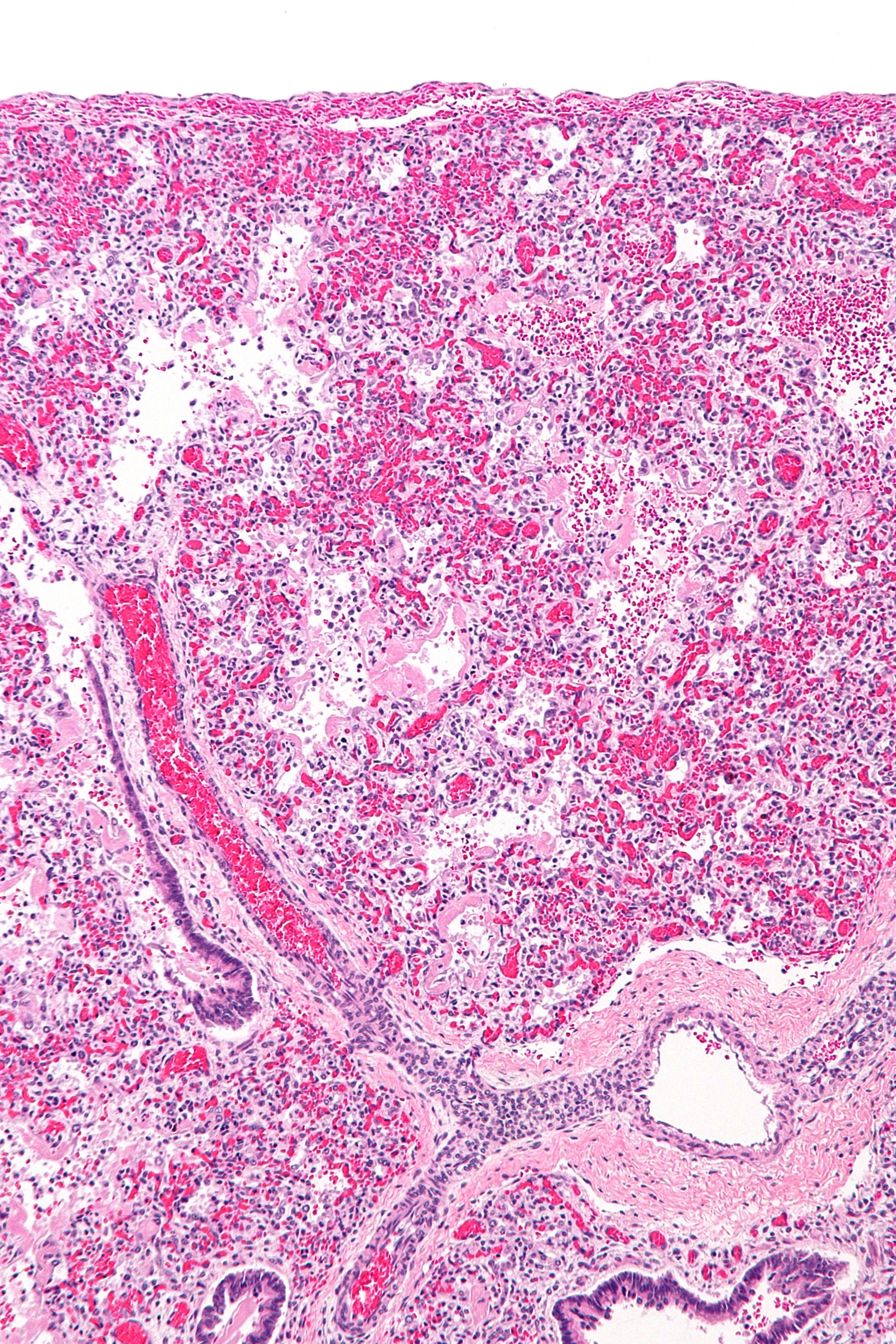
Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) is a type of respiratory failure characterized by rapid onset of widespread inflammation in the lungs. Symptoms include shortness of breath (dyspnea), rapid breathing (tachypnea), and bluish skin coloration (cyanosis). For those who survive, a decreased quality of life is common. Causes may include sepsis, pancreatitis, trauma, pneumonia, and aspiration. The underlying mechanism involves diffuse injury to cells which form the barrier of the microscopic air sacs of the lungs, surfactant dysfunction, activation of the immune system, and dysfunction of the body's regulation of blood clotting. In effect, ARDS impairs the lungs' ability to exchange oxygen and carbon dioxide. Adult diagnosis is based on a PaO2/FiO2 ratio (ratio of partial pressure arterial oxygen and fraction of inspired oxygen) of less than 300 mm Hg despite a positive end-expiratory pressure (PEEP) of more than 5 cm H2O. Cardiogenic pulmonary edema, as t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothelial
The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells form the barrier between vessels and tissue and control the flow of substances and fluid into and out of a tissue. Endothelial cells in direct contact with blood are called vascular endothelial cells whereas those in direct contact with lymph are known as lymphatic endothelial cells. Vascular endothelial cells line the entire circulatory system, from the heart to the smallest capillaries. These cells have unique functions that include fluid filtration, such as in the glomerulus of the kidney, blood vessel tone, hemostasis, neutrophil recruitment, and hormone trafficking. Endothelium of the interior surfaces of the heart chambers is called endocardium. An impaired function can lead to serious health issues throug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin Light Chain Kinase Regulation + Structural Motifs
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in '' Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho-associated Protein Kinase
Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC (PKA/ PKG/PKC) family of serine-threonine specific protein kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton. ROCKs ( ROCK1 and ROCK2) occur in mammals (human, rat, mouse, cow), zebrafish, ''Xenopus'', invertebrates ('' C. elegans'', mosquito, '' Drosophila'') and chicken. Human ROCK1 has a molecular mass of 158 kDa and is a major downstream effector of the small GTPase RhoA. Mammalian ROCK consists of a kinase domain, a coiled-coil region and a Pleckstrin homology (PH) domain, which reduces the kinase activity of ROCKs by an autoinhibitory intramolecular fold if RhoA-GTP is not present. Rat ROCKs were discovered as the first effectors of Rho and they induce the formation of stress fibers and focal adhesions by phosphorylating MLC (myosin light chain). Due to this phosphorylation, the actin binding of myosin II and, thus, the contractility increa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades. In biochemistry, the PKC family consists of fifteen isozymes in humans. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, DAG, and a phospholipid such as phosphatidylserine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin Light-chain Phosphatase
Myosin light-chain phosphatase, also called myosin phosphatase (EC 3.1.3.53; systematic name yosin-light-chainphosphate phosphohydrolase), is an enzyme (specifically a serine/threonine-specific protein phosphatase) that dephosphorylates the regulatory light chain of myosin II: : yosin light-chainphosphate + H2O = yosin light-chain+ phosphate This dephosphorylation reaction occurs in smooth muscle tissue and initiates the relaxation process of the muscle cells. Thus, myosin phosphatase undoes the muscle contraction process initiated by myosin light-chain kinase. The enzyme is composed of three subunits: the catalytic region (protein phosphatase 1, or PP1), the myosin binding subunit (MYPT1), and a third subunit (M20) of unknown function. The catalytic region uses two manganese ions as catalysts to dephosphorylate the light-chains on myosin, which causes a conformational change in the myosin and relaxes the muscle. The enzyme is highly conserved and is found in all organis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Striated Muscle
Striations means a series of ridges, furrows or linear marks, and is used in several ways: * Glacial striation * Striation (fatigue), in material * Striation (geology), a ''striation'' as a result of a geological fault * Striation Valley, in Antarctica * In hyperbolic geometry, a ''striation'' is a reflection across two parallel mirrors. * In anatomy, striated muscle * Striations can be found in certain glasses. These have been caused by turbulent flow during teeming (pouring) of the glass. * Striations can be observed in clouds In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid drop (liquid), droplets, ice crystals, frozen crystals, or other particulates, particles suspended in the atmosphere of a planetary body or similar space. .... See Barber's pole. * Ballistic fingerprinting {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troponin
image:Troponin Ribbon Diagram.png, 400px, Ribbon representation of the human cardiac troponin core complex (52 kDa core) in the calcium-saturated form. Blue = troponin C; green = troponin I; magenta = troponin T.; ; rendered with PyMOL Troponin, or the troponin complex, is a complex of three regulatory proteins (troponin C, troponin I, and troponin T) that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low. Function Troponin is attached to the protein tropomyosin and lies within the groove between actin filaments in muscle tissue. In a relaxed muscle, tropomyosin blocks the attachment site fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |