|
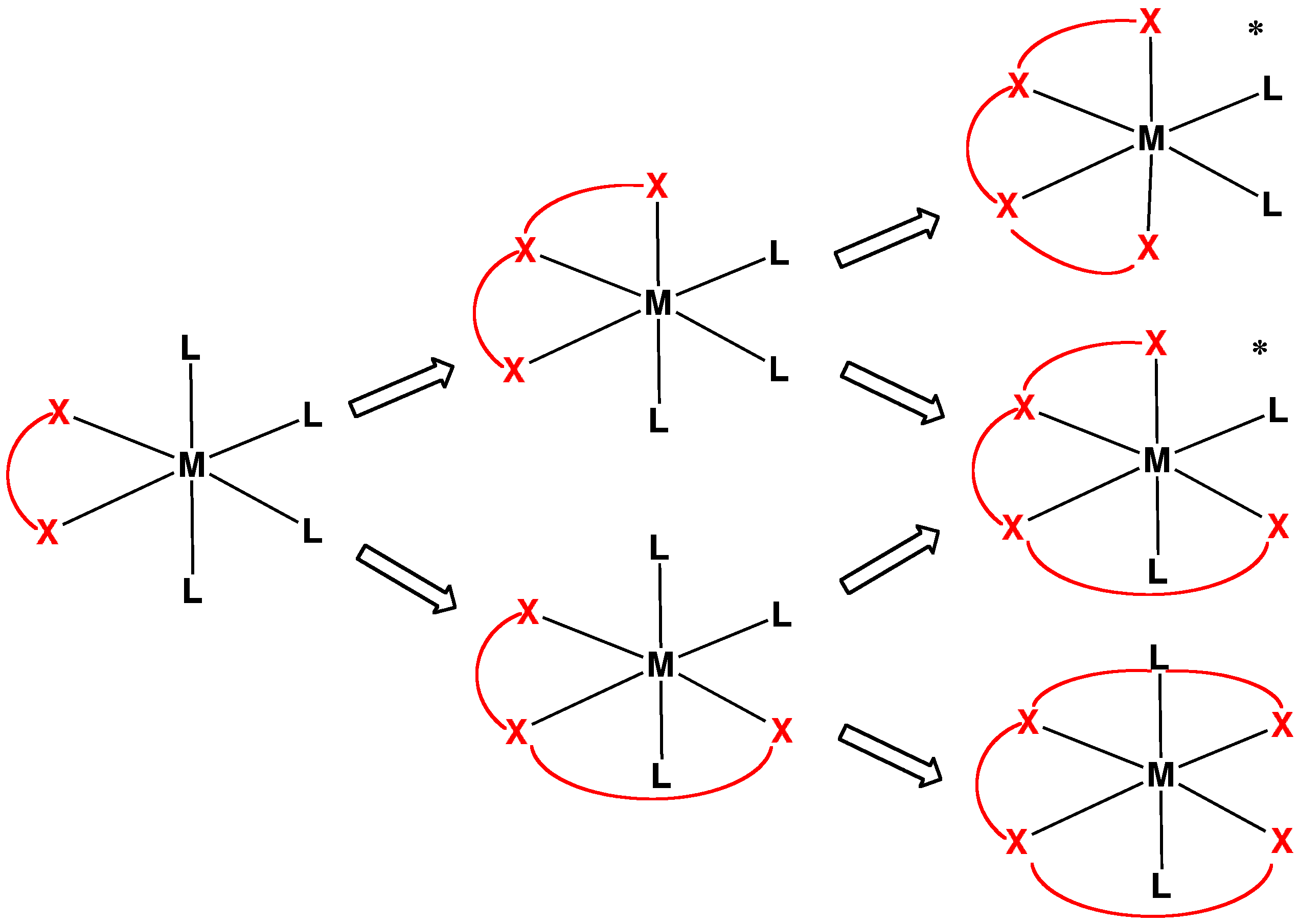
Monodentate
In coordination chemistry, denticity () refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be unidentate or monodentate. Ligands with more than one bonded atom are called multidentate or polydentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms. Denticity is different from hapticity because hapticity refers exclusively to ligands where the coordinating atoms are contiguous. In these cases the η ('eta') notation is used. Bridging ligands use the μ ('mu') notation. Classes Polydentate ligands are chelating agents and classified by their denticity. Some atoms cannot form the maximum possible number of bonds a ligand could make. In that case one or more binding sites ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in chela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetradentate Ligand
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands. Tetradentate ligands are common in nature in the form of chlorophyll, which has a core ligand called chlorin, and heme, which has a core ligand called porphyrin. They are responsible for the colour observed in plants and humans. Phthalocyanine is an artificial macrocyclic tetradentate ligand that is used to make blue and green pigments. Shape Tetradentate ligands can be classified by the topology of the connections between donor atoms. Common forms are linear (also called sequential), ring or tripodal. A tetrapodal ligand that is also tetradentate has four legs with donor atoms and a bridgehead that is not a donor. Upon binding with a central atom, there are several arrangements possible (known as geometric isomers). Linear ligands A linear tetradentate ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaliplatin
Oxaliplatin, sold under the brand name Eloxatin among others, is a cancer medication (platinum-based antineoplastic class) used to treat colorectal cancer. It is given by intravenous, infusion into a vein. Common side effects include paresthesia, numbness, feeling tired, nausea, diarrhea, and cytopenia, low blood cell counts. Other serious side effects include allergic reactions. Use in pregnancy is known to harm the baby. Oxaliplatin is in the platinum-based antineoplastic family of medications. It is believed to work by blocking the duplication of DNA. Oxaliplatin was patented in 1976 in Japan and approved for medical use in 1996 in Europe. It is on the 2023 WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Medical uses Oxaliplatin is used for treatment of colorectal cancer, typically along with folinic acid (leucovorin) and fluorouracil in a combination known as FOLFOX or along with capecitabine in a combination known as CA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapticity
In coordination chemistry, hapticity is the coordination complex, coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter eta (letter), η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the denticity, κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with mu (letter), μ ('mu'), which relates to bridging ligands. History The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Leslie Orgel, Orgel, and Rich described the structure of the "sandwich compound, sandwich complex" ferrocene by X-ray ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CSD CIF KOLGIB
CSD may refer to: Finance * Central securities depository * Confederate States Dollar * Serbian dinar, by previous ISO 4217 code Organizations Education * California School for the Deaf (other), several institutions * Canyons School District, in Utah, US * Cheltenham School District, in Pennsylvania, US * Christina School District, in Delaware, US * Cleveland School District, in Mississippi, US * Cordova School District, in Alaska, US Other organizations * Canteen Stores Department (India), a chain of stores operated by the Indian Ministry of Defence at military bases * CSD Pakistan (Canteen Stores Department), a chain of stores operated by the Pakistani Ministry of Defence * Chartered Society of Designers, a British learned society for various kinds of design work * Commission on Sustainable Development (1992–2013), a former UN agency * Communication Service for the Deaf, an American non-profit company providing ASL services * Congress of Democratic Trade Union ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EDTA
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula . This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly. Uses EDTA is widely used in industry. It also has applications in food preservation, medicine, cosmetics, water softening, in laboratories, and other fields. Industrial EDTA is mainly used to sequester (bind or confine) metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp and paper industry, EDTA inhibits the ability of metal ions, especiall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexadentate Ligand
A hexadentate ligand in coordination chemistry is a ligand that combines with a central metal atom with six bonds. One example of a hexadentate ligand that can form complexes with soft metal ions is TPEN. A commercially important hexadentate ligand is EDTA. The denticity of hexadentate ligands is often denoted with the prefix κ6. Topology The way the donor atoms are joined together in the molecule is its topology. Some topologies are simple, such as the linear or ring shapes. The molecule can also be branched, either at a donor atom, or at a non-donor atom. Example shapes are the tripod, and amplector, with a bifurcation at each end. Rigid molecules can be used to force unusual coordination such as trigonal prism In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc .... F. Lions identif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |