|
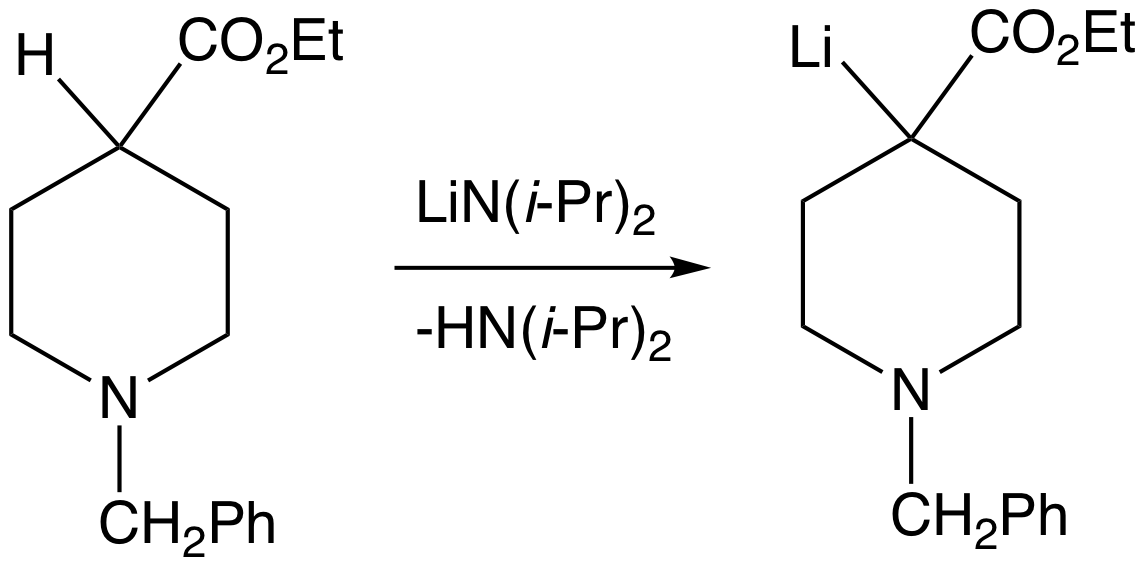
Methyl Anion
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group General Formulae V
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom chemical bond, bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In chemical formula, formulas, the group is often skeletal formula#Pseudoelement symbols, abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (chemistry), radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), usually denoted by H+, to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, is deprotonation.) Some examples include * The protonation of water by sulfuric acid: *: H2SO4 + H2O H3O+ + * The protonation of isobutene in the formation of a carbocation: *: (CH3)2C=CH2 + HBF4 (CH3)3C+ + * The protonation of ammonia in the formation of ammonium chloride from ammonia and hydrogen chloride: *: NH3( g) + HCl( g) → NH4Cl( s) Protonation is a fundamental chemical reaction and is a step in many stoichiometric and catalytic processes. Some ions and molecules can undergo more than one protonation and are labeled polybasic, which is true of many biological macromolecules. Protonation and deprotonation (removal of a proton) occur in most acid–base reactions; they are the core of most acid–base r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009. Definitions Generically IUPAC defines a superbase as a "compound having a very high basicity, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic and organometallic. Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge (1,8-bis(dimethylamino)naphthalene, pKBH+ = 18.6 in acetonitrile). In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mol (unit)
The mole (symbol mol) is a unit of measurement, the base unit in the International System of Units (SI) for ''amount of substance'', an SI base quantity proportional to the number of elementary entities of a substance. One mole is an aggregate of exactly elementary entities (approximately 602 sextillion or 602 billion times a trillion), which can be atoms, molecules, ions, ion pairs, or other particles. The number of particles in a mole is the Avogadro number (symbol ) and the numerical value of the '' Avogadro constant'' (symbol ) expressed in mol−1. The relationship between the mole, Avogadro number, and Avogadro constant can be expressed in the following equation:1\text = \frac = \frac The current SI value of the mole is based on the historical definition of the mole as the amount of substance that corresponds to the number of atoms in 12 grams of 12C, which made the molar mass of a compound in grams per mole, numerically equal to the average molecular mass or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kilojoule
The joule ( , or ; symbol: J) is the unit of energy in the International System of Units (SI). In terms of SI base units, one joule corresponds to one kilogram- metre squared per second squared One joule is equal to the amount of work done when a force of one newton displaces a body through a distance of one metre in the direction of that force. It is also the energy dissipated as heat when an electric current of one ampere passes through a resistance of one ohm for one second. It is named after the English physicist James Prescott Joule (1818–1889). Definition According to the International Bureau of Weights and Measures the joule is defined as "the work done when the point of application of 1 MKS unit of force ewtonmoves a distance of 1 metre in the direction of the force." In terms of SI base units and in terms of SI derived units with special names, the joule is defined as One joule is also equivalent to any of the following: * The work required to move ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Reaction
The standard enthalpy of reaction (denoted \Delta H_^\ominus) for a chemical reaction is the difference between total product and total reactant molar enthalpies, calculated for substances in their standard states. The value can be approximately interpreted in terms of the total of the chemical bond energies for bonds broken and bonds formed. For a generic chemical reaction :\nu_ \text + \nu_ \text ~+ ~... \rightarrow \nu_ \text + \nu_ \text ~+ ~... the standard enthalpy of reaction \Delta H_^\ominus is related to the standard enthalpy of formation \Delta_ H^\ominus values of the reactants and products by the following equation: : \Delta H_^\ominus = \sum_ \nu_p\Delta_ H_^ - \sum_ \nu_r\Delta_ H_^ In this equation, \nu_i are the stoichiometric coefficients of each product and reactant. The standard enthalpy of formation, which has been determined for a vast number of substances, is the change of enthalpy during the formation of 1 mole of the substance from its constitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Torr
The torr (symbol: Torr) is a Pressure#Units, unit of pressure based on an absolute scale, defined as exactly of a standard atmosphere (unit), atmosphere (101325 Pa). Thus one torr is exactly (≈ ). Historically, one torr was intended to be the same as one "millimetre of mercury", but subsequent redefinitions of the two units of measurement, units made the torr marginally lower (by less than 0.000015%). The torr is not part of the International System of Units (SI). Even so, it is often combined with the metric prefix milli to name one millitorr (mTorr), equal to 0.001 Torr. The unit was named after Evangelista Torricelli, an Italian physicist and mathematician who discovered the principle of the barometer in 1644. Nomenclature and common errors The unit name ''torr'' is written in letter case, lower case, while its symbol ("Torr") is always written with an uppercase initial; including in combinations with prefixes and other unit symbols, as in "mTorr" (millitorr) or " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
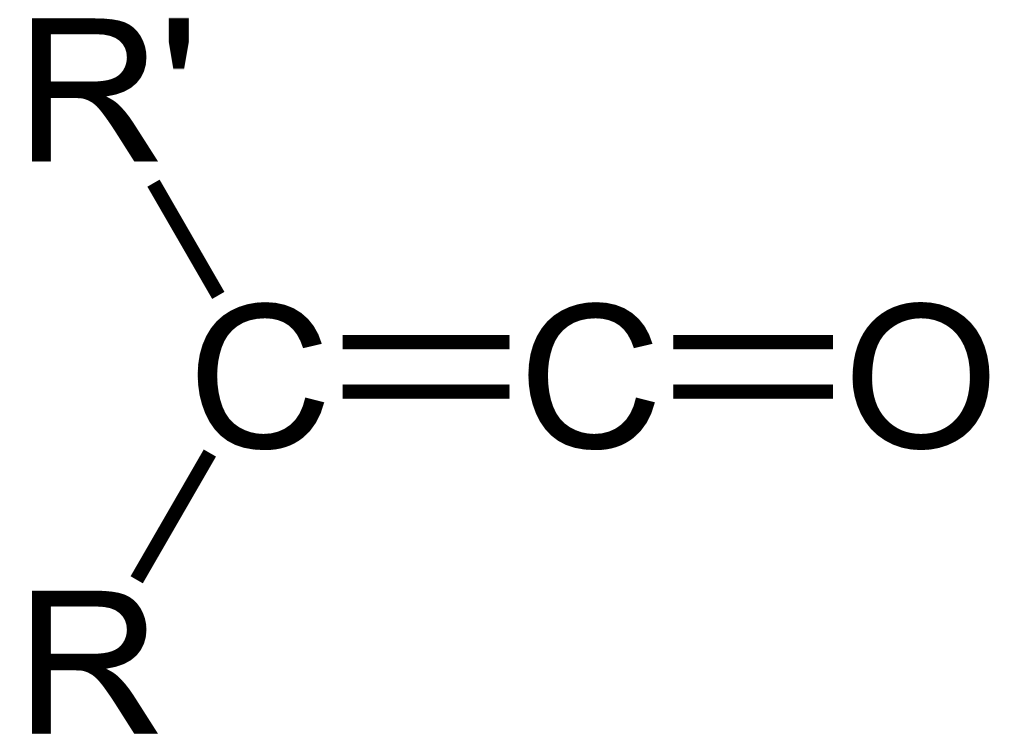
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features Peer review, peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2022 ''Journal Citation Reports'' (with an ascribed impact factor of 50.5), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in the autumn of 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander MacMillan (publisher), Alexander MacMillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mashable
Mashable is a Online newspaper, news website, digital media platform and entertainment company founded by Pete Cashmore in 2005. History Mashable was founded by Pete Cashmore while living in Aberdeen, Scotland, in July 2004. Early iterations of the site were a simple WordPress blog, with Cashmore as sole author. Fame came relatively quickly, with ''Time (magazine), Time'' magazine noting Mashable as one of the 25 best blogs of 2009. it had over 6,000,000 Twitter followers and over 3,200,000 fans on Facebook. In June 2016, it acquired YouTube channel CineFix from Whalerock Industries. In December 2017, Ziff Davis bought Mashable for $50 million, a price described by ''Recode'' as a "fire sale" price. Mashable had not been meeting its advertising targets, accumulating $4.2 million in losses in the quarter ending September 2017. After the sale, Mashable laid off 50 staff, but preserved top management. Under Ziff Davis, Mashable has grown and expanded to many countries in multiple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged Atomic nucleus, atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as Alcohol (chemistry), alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |