|
Medicines
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in many ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the medical prescription) from over-the-counter drugs (those that consumers can order for themselves). Medicines may be classified by mode of action, route of administration, biological system affected, or therapeutic effects. The World Health Organization keeps a list of essential medicines. Drug discovery and drug development are complex and expensive endeavors undertaken by pharmaceutical companies, academic scientists, and governments. As a resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Medicines
Essential medicines, as defined by the World Health Organization (WHO), are medicines that "''satisfy the priority health care needs of the population''". Essential medicines should be accessible to people at all times, in sufficient amounts, and be generally affordable. Since 1977, the WHO has published a model list of essential medicines, with the 2019 list for adult patients containing over 400 medicines. Since 2007, a separate list of medicines intended for child patients has been published. A new list was published in 2021, for both adults and children. Several changes have been implemented since the 2021 edition, including that medication cost should not be grounds for exclusion criteria if it meets other selection criteria, and cost-effectiveness differences should be evaluated within therapeutic areas. The following year, antiretroviral agents, usually used in the treatment of HIV/AIDS, were included on the list of essential medicines. The WHO distinguishes betwe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
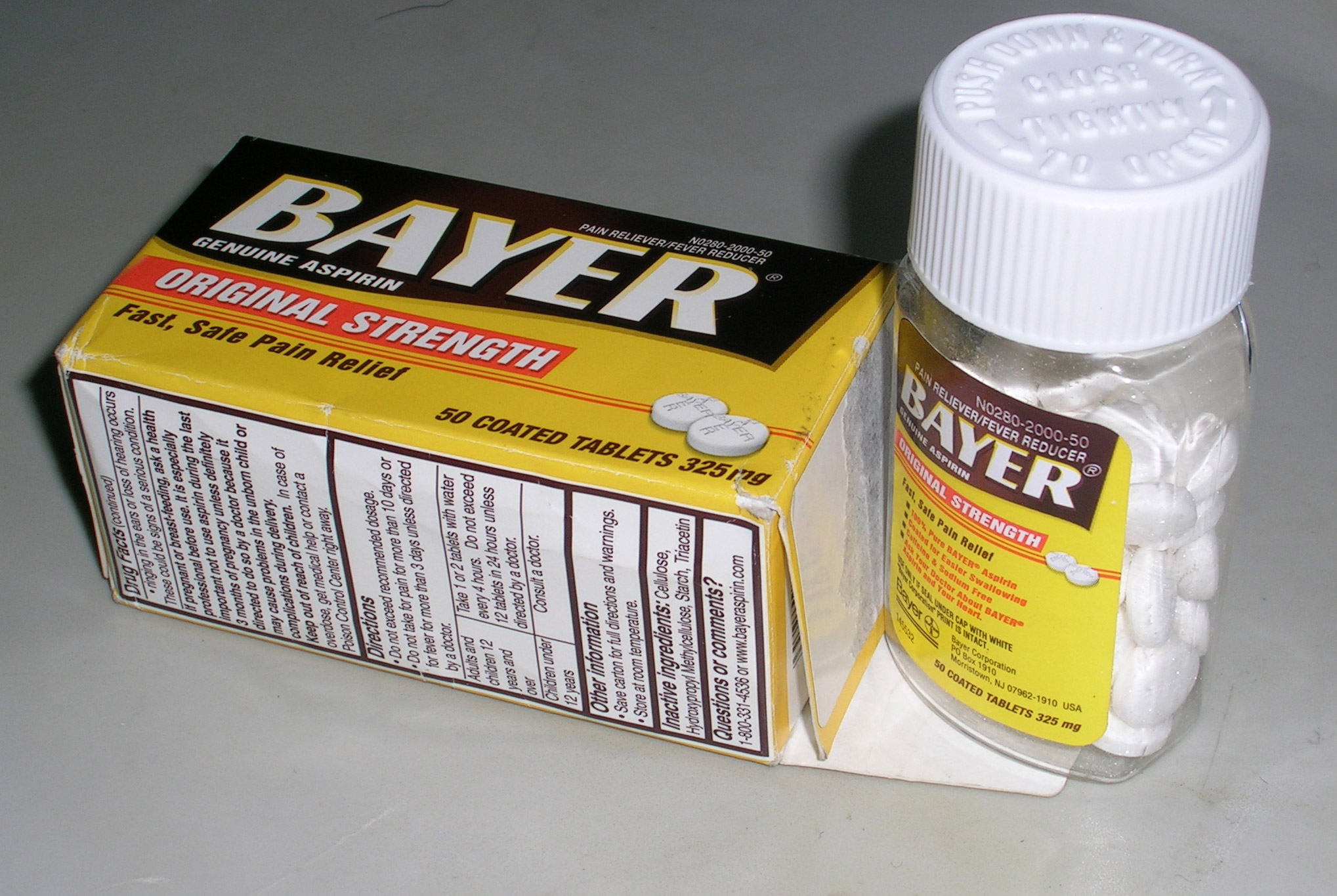
Over-the-counter Drug
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) and strengths of final products. The term ''over-the-counter'' (''OTC'') refers to a medication that can be purchased without a medical prescription. In contrast, prescription drugs require a prescription from a doctor or other health care professional and should only be used by the prescribed individual. Some drugs may be legally classified as over-the-counter (i.e. no prescription is required), but may only be dispensed by a pharmacist after an assessment of the patient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Industry
The pharmaceutical industry is a medical industry that discovers, develops, produces, and markets pharmaceutical goods such as medications and medical devices. Medications are then administered to (or self-administered by) patients for curing or preventing disease or for alleviating symptoms of illness or injury. Pharmaceutical companies may deal in generic drugs, branded drugs, or both, in different contexts. Generic materials are without the involvement of intellectual property, whereas branded materials are protected by chemical patents. The industry's various subdivisions include distinct areas, such as manufacturing biologics and total synthesis. The industry is subject to a variety of laws and regulations that govern the patenting, efficacy testing, safety evaluation, and marketing of these drugs. The global pharmaceutical market produced treatments worth a total of $1,228.45 billion in 2020. The sector showed a compound annual growth rate (CAGR) of 1.8% in 2021, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug injection, injection, smoking, ingestion, absorption (skin), absorption via a dermal patch, patch on the skin, suppository, or sublingual administration, dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to pharmacotherapy, treat, cure, preventive healthcare, prevent, or medical diagnosis, diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prescription Drug
A prescription drug (also prescription medication, prescription medicine or prescription-only medication) is a pharmaceutical drug that is permitted to be dispensed only to those with a medical prescription. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practising medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug. In North America, , usually printed as "Rx", is used as an abbreviation of the word "prescription". It is a contraction of the Latin word "''recipe''" (an imperative form of "recipere") meaning "take". Prescription drugs are often dispensed together with a monograph (in Europe, a Patient Information Leaflet or PIL) that gives detailed information about the drug. The use of prescription drugs has been increasing since the 1960s. Regul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacology
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals. The field encompasses drug composition and properties, functions, sources, synthesis and drug design, molecular and cellular mechanisms, organ/systems mechanisms, signal transduction/cellular communication, molecular diagnostics, interactions, chemical biology, therapy, and medical applications and antipathogenic capabilities. The two main areas of pharmacology are pharmacodynamics and pharmacokinetics. Pharmacodynamics studies the effects of a drug on biological systems, and pharmacokinetics studies the effects of biological systems on a drug. In broad terms, pharmacod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Prescription
A prescription, often abbreviated or Rx, is a formal communication from physicians or other registered healthcare professionals to a pharmacist, authorizing them to dispense a specific prescription drug for a specific patient. Historically, it was a physician's instruction to an apothecary listing the materials to be compounded into a treatmentthe symbol (a capital letter R, crossed to indicate abbreviation) comes from the first word of a medieval prescription, Latin (), that gave the list of the materials to be compounded. Format and definition For a communication to be accepted as a legal medical prescription, it needs to be filed by a qualified dentist, advanced practice nurse, physician, or veterinarian, for whom the medication prescribed is within their scope of practice to prescribe. This is regardless of whether the prescription includes prescription drugs, controlled substances, or over-the-counter treatments. Prescriptions may be entered into an electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Pricing
Medication costs, also known as drug costs are a common health care cost for many people and health care systems. Prescription costs are the costs to the end consumer. Medication costs are influenced by multiple factors such as patents, stakeholder influence, and marketing expenses. A number of countries including Canada, parts of Europe, and Brazil use external reference pricing as a means to compare drug prices and to determine a base price for a particular medication. Other countries use pharmacoeconomics, which looks at the cost/benefit of a product in terms of quality of life, alternative treatments (drug and non-drug), and cost reduction or avoidance in other parts of the health care system (for example, a drug may reduce the need for a surgical intervention, thereby saving money). Structures like the UK's National Institute for Health and Clinical Excellence and to a lesser extent Canada's Common Drug Review (a division of the Canadian Agency for Drugs and Technologies in Hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. These h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Discovery
In the fields of medicine, biotechnology, and pharmacology, drug discovery is the process by which new candidate medications are discovered. Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by serendipitous discovery, as with penicillin. More recently, chemical libraries of synthetic small molecules, natural products, or extracts were screened in intact cells or whole organisms to identify substances that had a desirable therapeutic effect in a process known as classical pharmacology. After sequencing of the human genome allowed rapid cloning and synthesis of large quantities of purified proteins, it has become common practice to use high throughput screening of large compounds libraries against isolated biological targets which are hypothesized to be disease-modifying in a process known as reverse pharmacology. Hits from these screens are then tested in cells and then in animals for efficacy. Modern drug discovery i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Geneva, Switzerland, and has 6 regional offices and 150 field offices worldwide. Only sovereign states are eligible to join, and it is the largest intergovernmental health organization at the international level. The WHO's purpose is to achieve the highest possible level of health for all the world's people, defining health as "a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity." The main functions of the World Health Organization include promoting the control of epidemic and endemic diseases; providing and improving the teaching and training in public health, the medical treatment of disease, and related matters; and promoting the establishment of international standards for biologic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |