|
List Of Types Of Equilibrium
{{Unreferenced, date=September 2022 This is a list of various types of equilibrium (other), equilibrium, the condition of a system in which all competing influences are balanced. Biology * Equilibrioception, the sense of a balance present in human beings and animals * Equilibrium unfolding, the process of unfolding a protein or RNA molecule by gradually changing its environment * Genetic equilibrium, theoretical state in which a population is not evolving * Homeostasis, the ability of an open system, especially living organisms, to regulate its internal environment * Punctuated equilibrium, theory in evolutionary biology * Sedimentation equilibrium, analytical ultracentrifugation method for measuring protein molecular masses in solution * Equilibrium Theory (Island biogeography), MacArthur-Wilson theory explaining biodiversity character of ecological islands * Osmotic equilibrium, balance between solvent flow and pressure across a membrane Physics * Equilibrant force, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equilibrium (other)
List of types of equilibrium, the condition of a system in which all competing influences are balanced, in a wide variety of contexts. Equilibrium may also refer to: Film and television * ''Equilibrium'' (film), a 2002 science fiction film * ''The Story of Three Loves'', also known as ''Equilibrium'', a 1953 romantic anthology film * "Equilibrium" (''seaQuest 2032'') * ''Equilibrium'', short film by Steven Soderbergh, a segment of ''Eros'' * "Equilibrium" (''Star Trek: Deep Space Nine''), Star Trek DS9 Episode 4, Season 3 Music * Equilibrium (band), a folk metal band from Germany * ''Equilibrium'' (Crowbar album), 2000 * ''Equilibrium'' (Erik Mongrain album), 2008 * ''Equilibrium'' (God Forbid album), 2012 * ''Equilibrium'' (Whitecross album), 1995 * ''Equilibrium'' (Matthew Shipp album), 2003 * ''IX Equilibrium ''IX Equilibrium'' is the third studio album by the black metal band Emperor (band), Emperor. It was released on March 15, 1999 in Europe and on April 27th, 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of matter nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, no macroscopic change occurs. Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, until disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly balanced microscopic exchanges occur; this is the physical explanation of the notion of macroscopic equilibrium. A thermody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
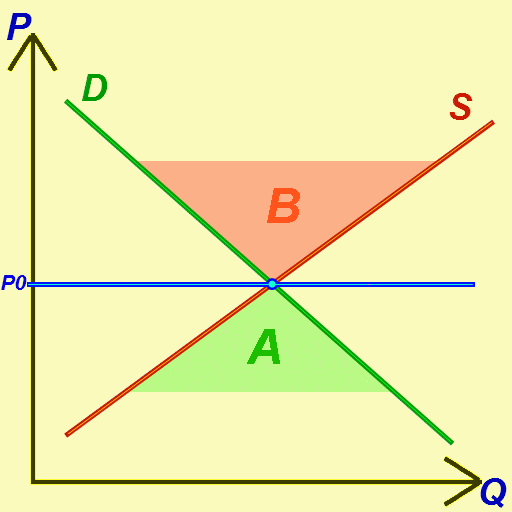
Economic Equilibrium
In economics, economic equilibrium is a situation in which economic forces such as supply and demand are balanced and in the absence of external influences the ( equilibrium) values of economic variables will not change. For example, in the standard text perfect competition, equilibrium occurs at the point at which quantity demanded and quantity supplied are equal. Market equilibrium in this case is a condition where a market price is established through competition such that the amount of goods or services sought by buyers is equal to the amount of goods or services produced by sellers. This price is often called the competitive price or market clearing price and will tend not to change unless demand or supply changes, and quantity is called the "competitive quantity" or market clearing quantity. But the concept of ''equilibrium'' in economics also applies to imperfectly competitive markets, where it takes the form of a Nash equilibrium. Understanding economic equili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Competitive Equilibrium
Competitive equilibrium (also called: Walrasian equilibrium) is a concept of economic equilibrium introduced by Kenneth Arrow and Gérard Debreu in 1951 appropriate for the analysis of commodity markets with flexible prices and many traders, and serving as the benchmark of efficiency in economic analysis. It relies crucially on the assumption of a competitive environment where each trader decides upon a quantity that is so small compared to the total quantity traded in the market that their individual transactions have no influence on the prices. Competitive markets are an ideal standard by which other market structures are evaluated. Definitions A competitive equilibrium (CE) consists of two elements: * A price function P. It takes as argument a vector representing a bundle of commodities, and returns a positive real number that represents its price. Usually the price function is linear - it is represented as a vector of prices, a price for each commodity type. * An allocation m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility Equilibrium
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent ''solubility product'' which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios. Definitions A solubility equilibrium exists when a chemical compound in the solid state is in chemical equilibrium with a solution containing the compound. This type of equilibrium is an example of dynamic equilibrium in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution and precipitation are equal to one another. When equilibrium is established and the solid has not all disso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schlenk Equilibrium
Schlenk can refer to: People * Wilhelm Schlenk (1879-1943), German chemist In chemistry * Schlenk flask * Schlenk line The Schlenk line (also vacuum gas manifold) is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is co .... or Schlenk apparatus * Schlenk equilibrium Other * Mepco Schlenk Engineering College {{disambig, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quasistatic Equilibrium
In thermodynamics, a quasi-static process (also known as a quasi-equilibrium process; from the Latin ''quasi'', meaning ‘as if’), is a thermodynamic process that happens slowly enough for the system to remain in internal physical (but not necessarily chemical) thermodynamic equilibrium. An example of this is quasi-static expansion of a mixture of hydrogen and oxygen gas, where the volume of the system changes so slowly that the pressure remains uniform throughout the system at each instant of time during the process. Such an idealized process is a succession of physical equilibrium states, characterized by infinite slowness.Rajput, R.K. (2010). ''A Textbook of Engineering Thermodynamics'', 4th edition, Laxmi Publications (P) Ltd, New Delhi, pages 21, 45, 58. Only in a quasi-static thermodynamic process can we exactly define intensive quantities (such as pressure, temperature, specific volume, specific entropy) of the system at every instant during the whole process; otherwi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partition Equilibrium
Partition equilibrium is a special case of chemical equilibrium. The most common chemical equilibrium systems involve reactants and products in the same phase - either all gases or all solutions. However, it is also possible to get equilibria between substances in different phases, such as two liquids that do not mix (are immiscible). Partition equilibria are described by Nernst's distribution law. Example For example, ammonia (NH3) is soluble in both water (aq) and the organic solvent trichloromethane (CHCl3) - two immiscible solvents. If ammonia is first dissolved in water, and then an equal volume of trichloromethane is added, and the two liquids shaken together, the following equilibrium is established: :Kc = H3 (CHCl3) H3 (aq) (where Kc is the equilibrium constant) The equilibrium concentrations of ammonia in each layer can be established by titration with standard acid solution. It can thus be determined that Kc remains constant, with a value ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equilibrium Constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant. A knowledge of equilibrium constants is essential for the understanding of many chemical systems, as well as biochemical processes such as oxygen transport by hemoglobin in blood and acid–base homeostasis in the human body. Stability constants, formation c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances transition between the reactants and products at equal rates, meaning there is no net change. Reactants and products are formed at such a rate that the concentration of neither changes. It is a particular example of a system in a steady state. In physics, concerning thermodynamics, a closed system is in thermodynamic equilibrium when reactions occur at such rates that the composition of the mixture does not change with time. Reactions do in fact occur, sometimes vigorously, but to such an extent that changes in composition cannot be observed. Equilibrium constants can be expressed in terms of the rate constants for reversible reactions. Examples In a new bottle of soda, the concentration of carbon dioxide in the liquid phase has a particular value. If half of the liquid is poured out and the bottle is sealed, carbon dioxide will leave the liquid phase at an ever-decreasing rate, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs–Donnan Effect
The Gibbs–Donnan effect (also known as the Donnan's effect, Donnan law, Donnan equilibrium, or Gibbs–Donnan equilibrium) is a name for the behaviour of charged particles near a semi-permeable membrane that sometimes fail to distribute evenly across the two sides of the membrane. The usual cause is the presence of a different charged substance that is unable to pass through the membrane and thus creates an uneven electrical charge. For example, the large anionic proteins in blood plasma are not permeable to capillary walls. Because small cations are attracted, but are not bound to the proteins, small anions will cross capillary walls away from the anionic proteins more readily than small cations. Thus, some ionic species can pass through the barrier while others cannot. The solutions may be gels or colloids as well as solutions of electrolytes, and as such the phase boundary between gels, or a gel and a liquid, can also act as a selective barrier. The electric potential ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |