|
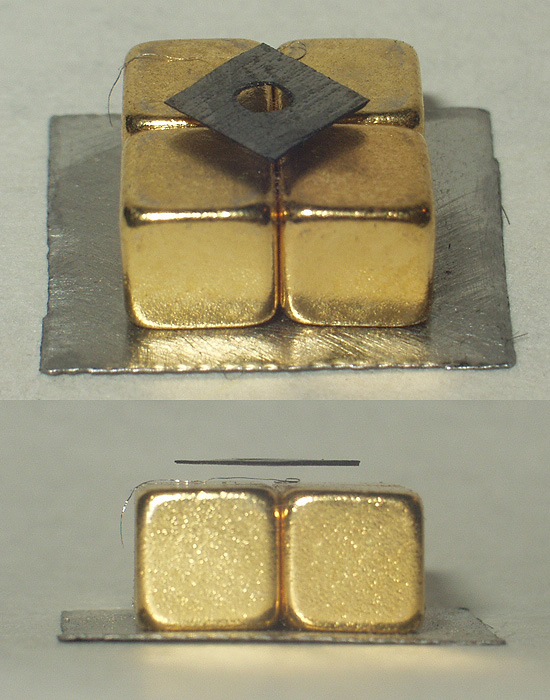
LiH
Lithium hydride is an inorganic compound with the formula Lithium, LiHydride, H. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a Hydride#Ionic hydrides, salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all Polar solvent, protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound. Physical properties LiH is a diamagnetism, diamagnetic and an Ionic conductivity (solid state), ionic conductor with a Electrical resistivity and conductivity, conductivity gradually increasing from at 443 °C to 0.18 Ω−1cm−1 at 754 °C; there is no discontinuity in this increase through the melting point. The dielectric constant of LiH decreases from 13.0 (static, low frequencies) to 3.6 (visible-light frequencies). LiH is a sof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Aluminium Hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage. Properties, structure, preparation LAH is a colourless solid but commercial samples are usually gray due to contamination. This material can be purified by recrystallization from diethyl ether. Large-scale purifications employ a Soxhlet extractor. Commonly, the impure gray material is used in synthesis, since the impurities are innocuous and can be easily separated from the organic products. The pure powdered material is pyrophoric, but not its large crystals. Some commercial materials contain mineral oil to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Borohydride
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.Luca Banfi, Enrica Narisano, Renata Riva, Ellen W. Baxter, "Lithium Borohydride" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons. . Preparation Lithium borohydride may be prepared by the metathesis reaction, which occurs upon ball-milling the more commonly available sodium borohydride and lithium bromide: : NaBH4 + LiBr → NaBr + LiBH4 Alternatively, it may be synthesized by treating boron trifluoride with lithium hydride in diethyl ether: : BF3 + 4 LiH → LiBH4 + 3 LiF Reactions Lithium borohydride is a stronger reducing agent than sodium borohydride. In mixtures of methanol and diethyl ether, lithium borohyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, and water. It is an ionic material that is insoluble in organic solvents (although soluble in molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid. Basic properties and structure NaH is produced by the direct reaction of hydrogen and liquid sodium.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Pure NaH is colorless, although samples generally appear grey. NaH is ca. 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Hydride
Caesium hydride or cesium hydride (CsH) is a compound of caesium and hydrogen. It is an alkali metal hydride. It was the first substance to be created by light-induced particle formation in metal vapor, and showed promise in early studies of an ion propulsion system using caesium. It is the most reactive stable alkaline metal hydride of all. It is a powerful superbase and reacts with water extremely vigorously. The caesium nuclei in CsH can be hyperpolarized through interactions with an optically pumped caesium vapor in a process known as spin-exchange optical pumping (SEOP). SEOP can increase the nuclear magnetic resonance (NMR) signal of caesium nuclei by an order of magnitude. It is very difficult to make caesium hydride in a pure form. Caesium hydride can be produced by heating caesium carbonate and metallic magnesium in hydrogen at 580 to 620 degrees Celsius. Crystal structure At room temperature and atmospheric pressure, CsH has the same structure as NaCl Sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Borohydride
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.Luca Banfi, Enrica Narisano, Renata Riva, Ellen W. Baxter, "Lithium Borohydride" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons. . Preparation Lithium borohydride may be prepared by the metathesis reaction, which occurs upon ball-milling the more commonly available sodium borohydride and lithium bromide: : NaBH4 + LiBr → NaBr + LiBH4 Alternatively, it may be synthesized by treating boron trifluoride with lithium hydride in diethyl ether: : BF3 + 4 LiH → LiBH4 + 3 LiF Reactions Lithium borohydride is a stronger reducing agent than sodium borohydride. In mixtures of methanol and diethyl ether, lithium borohyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, and water. It is an ionic material that is insoluble in organic solvents (although soluble in molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid. Basic properties and structure NaH is produced by the direct reaction of hydrogen and liquid sodium.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Pure NaH is colorless, although samples generally appear grey. NaH is ca. 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Conductivity (solid State)
Ionic conductivity (denoted by ) is a measure of a substance's tendency towards ionic conduction. Ionic conduction is the movement of ions. The phenomenon is observed in solids and solutions. Ionic conduction is one mechanism of current. In crystalline solids In most solids, ions rigidly occupy fixed positions, strongly embraced by neighboring atoms or ions. In some solids, selected ions are highly mobile allowing ionic conduction. The mobility increases with temperature. Materials exhibiting this property are used in batteries. A well-known ion conductive solid is β''-alumina ("BASE"), a form of aluminium oxide that has channels through which sodium cations can hop. When this ceramic is complexed with a mobile ion, such as Na+, it behaves as so-called fast ion conductor. BASE is used as a membrane in several types of molten salt electrochemical cell. In glasses Ion conduction in disordered solids like glasses, polymers, nanocomposites, defective crystals and other di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetism
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid, that transitions to white with decreasing crystal size. Although odorless, lithium fluoride has a bitter-saline taste. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Formation of LiF from the elements releases one of the highest energy per mass of reactants, second only to that of BeO. Manufacturing LiF is prepared from lithium hydroxide or lithium carbonate with hydrogen fluoride. Applications Precursor to LiPF6 for batteries Lithium fluoride is reacted with hydrogen fluoride (HF) and phosphorus pentachloride to make lithium hexafluorophosphate, an ingredient in lithium ion battery electrolyte. In molten salts Fluorine is produced by the electrolysis of molten potassium bifluoride. This electrolysis proceeds more efficiently when the electrolyte contains a few percent of LiF, possibl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |