|
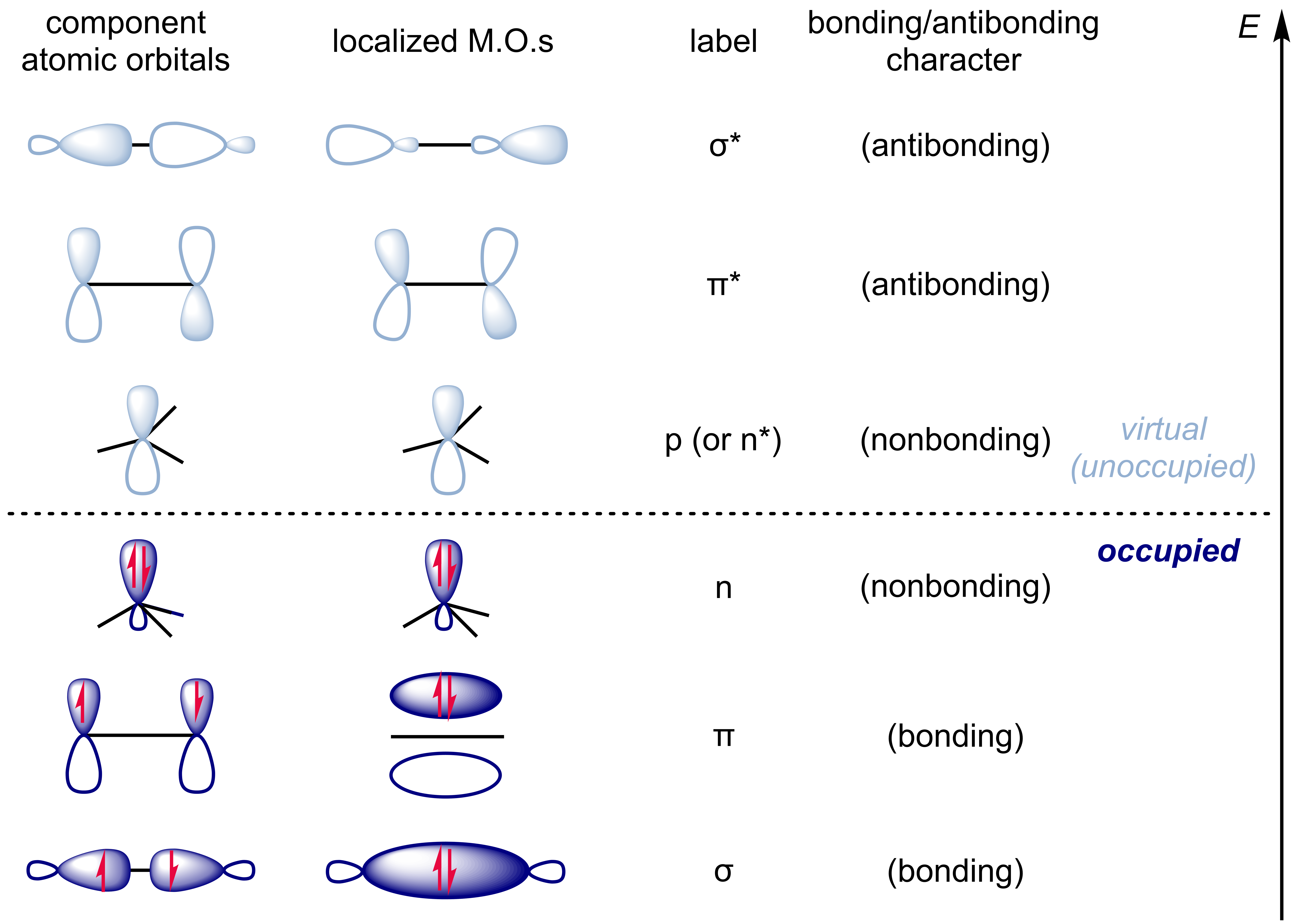
Localized Molecular Orbitals
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The local ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule by forming a valence chemical bond, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slater Determinant
In quantum mechanics, a Slater determinant is an expression that describes the wave function of a multi-fermionic system. It satisfies anti-symmetry requirements, and consequently the Pauli principle, by changing sign upon exchange of two fermions.Molecular Quantum Mechanics Parts I and II: An Introduction to QUANTUM CHEMISTRY (Volume 1), P. W. Atkins, Oxford University Press, 1977, . Only a small subset of all possible many-body fermionic wave functions can be written as a single Slater determinant, but those form an important and useful subset because of their simplicity. The Slater determinant arises from the consideration of a wave function for a collection of electrons, each with a wave function known as the spin-orbital \chi(\mathbf), where \mathbf denotes the position and spin of a single electron. A Slater determinant containing two electrons with the same spin orbital would correspond to a wave function that is zero everywhere. The Slater determinant is named for John ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of Molecule, molecules, Material, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed Wave function, wave functions as well as to observable properties such as structures, spectra, and Thermodynamics, thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics. Chemists rely heavily on spectroscopy through which information regarding the Quantization (physics), quantization of energy on a molecular scale can be obtained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-pi And Equivalent-orbital Models
The σ-π model and equivalent-orbital model refer to two possible representations of molecules in valence bond theory. The σ-π model differentiates bonds and lone pairs of σ symmetry from those of π symmetry, while the equivalent-orbital model hybridizes them. The σ-π treatment takes into account molecular symmetry and is better suited to interpretation of aromatic molecules (Hückel's rule), although computational calculations of certain molecules tend to optimize better under the equivalent-orbital treatment. The two representations produce the same total electron density and are related by a unitary transformation of the occupied molecular orbitals; different localization procedures yield either of the two. Two equivalent orbitals ''h'' and ''h''' can be constructed by taking linear combinations ''h'' = ''c''1σ + ''c''2π and ''h''' = ''c''1σ – ''c''2π for an appropriate choice of coefficients ''c''1 and ''c''2. In a 1996 review, Kenneth B. Wiberg concluded that "al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene radical, methylene, the parent hydride from which all other carbene compounds are formally derived. There are two types of carbenes: singlet state, singlets or triplet state, triplets, depending upon their electronic structure. The different classes undergo different reactions. Most carbenes are extremely reactive and short-lived. A small number (the diHalogen, halocarbenes, carbon monoxide, and carbon monosulfide) can be isolated, and can stabilize as Coordination complex, metal ligands, but otherwise cannot be stored in bulk. A rare exception are the persistent carbenes, which have extensive application in modern organometallic chemistry. Generatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pair, Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the Molecular geometry, shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoelectronic Effect
In chemistry, primarily Organic chemistry, organic and computational chemistry, a stereoelectronic effectAlabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-1118906349.html is an effect on molecular structure, molecular geometry, chemical reactivity, reactivity, or physical properties due to spatial relationships in the molecules' electronic structures, electronic structure, in particular the interaction between atomic orbital, atomic and/or molecular orbitals. Phrased differently, stereoelectronic effects can also be defined as the geometric constraints placed on the Ground state, ground and/or transition states of molecules that arise from considerations of orbital overlap. Thus, a stereoelectronic effect explains a particular molecular property or reactivity by invoking stabilizing or destabilizing interactions that depend on the relative orientation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hückel Molecular Orbital Theory (named after Erich Hückel), a method of determining aromaticity in organic molecules
* (1895-1973), German chemist
* (born 1936), German diplomat, Ambassador of the GDR in Chad
{{disambig ...
Hückel or Huckel may refer to: * Erich Hückel (1896-1980), German physicist and chemist ** Debye–Hückel equation (named after Peter Debye and Erich Hückel), in chemistry, a method of calculating activity coefficients ** Hückel method (named after Erich Hückel), a method for the determination of energies of molecular orbitals *** Extended Hückel method, considers also sigma orbitals (whereas the original Hückel method only considers pi orbitals) ** Hückel's rule In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Localized MOs
Local may refer to: Geography and transportation * Local (train), a train serving local traffic demand * Local, Missouri, a community in the United States Arts, entertainment, and media * ''Local'' (comics), a limited series comic book by Brian Wood and Ryan Kelly * ''Local'' (novel), a 2001 novel by Jaideep Varma * ''The Local'' (film), a 2008 action-drama film * ''The Local'', English-language news websites in several European countries Computing * .local, a network address component Mathematics * Local property, a property which occurs on ''sufficiently small'' or ''arbitrarily small'' neighborhoods of points * Local ring, type of ring in commutative algebra Other uses * Pub, a drinking establishment, known as a "local" to its regulars See also * * * Local group (other) * Locale (other) * Localism (other) * Locality (other) * Localization (other) * Locus (other) * Lokal (other) Lokal may refer to: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
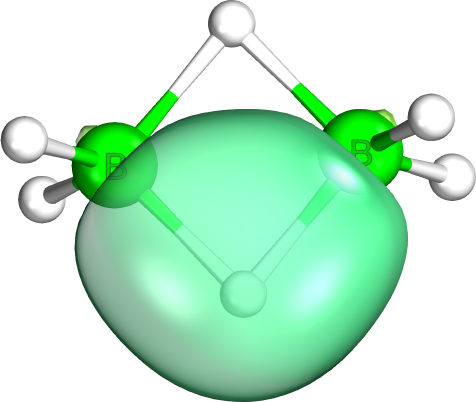
Intrinsic Bond Orbitals
Intrinsic bond orbitals (IBO) are localized molecular orbitals giving exact and non-empirical representations of Wave function, wave functions. They are obtained by unitary transformation and form an orthogonal set of orbitals localized on a minimal number of atoms. IBOs present an intuitive and unbiased interpretation of chemical bonding with naturally arising Lewis structure, Lewis structures. For this reason IBOs have been successfully employed for the elucidation of molecular structures and electron flow along the intrinsic reaction coordinate (IRC). IBOs have also found application as Wannier function, Wannier functions in the study of solids. Theory The IBO method entails molecular wave-functions calculated using self-consistent field (SCF) methods such as Kohn-Sham density functional theory (DFT) which are expressed as linear combinations of localized molecular orbitals. In order to arrive at IBOs, intrinsic atomic orbitals (IAOs) are first calculated as representations of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |