|
Intrinsic Bond Orbitals
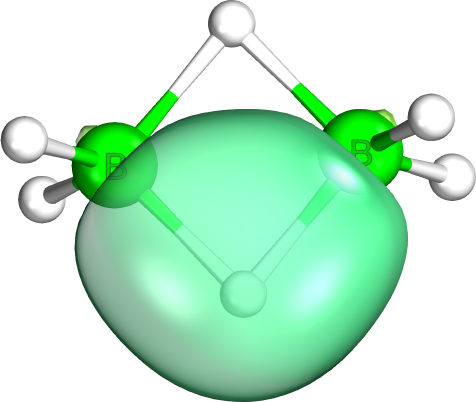
Intrinsic bond orbitals (IBO) are localized molecular orbitals giving exact and non-empirical representations of Wave function, wave functions. They are obtained by unitary transformation and form an orthogonal set of orbitals localized on a minimal number of atoms. IBOs present an intuitive and unbiased interpretation of chemical bonding with naturally arising Lewis structure, Lewis structures. For this reason IBOs have been successfully employed for the elucidation of molecular structures and electron flow along the intrinsic reaction coordinate (IRC). IBOs have also found application as Wannier function, Wannier functions in the study of solids. Theory The IBO method entails molecular wave-functions calculated using self-consistent field (SCF) methods such as Kohn-Sham density functional theory (DFT) which are expressed as linear combinations of localized molecular orbitals. In order to arrive at IBOs, intrinsic atomic orbitals (IAOs) are first calculated as representations of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Localized Molecular Orbitals
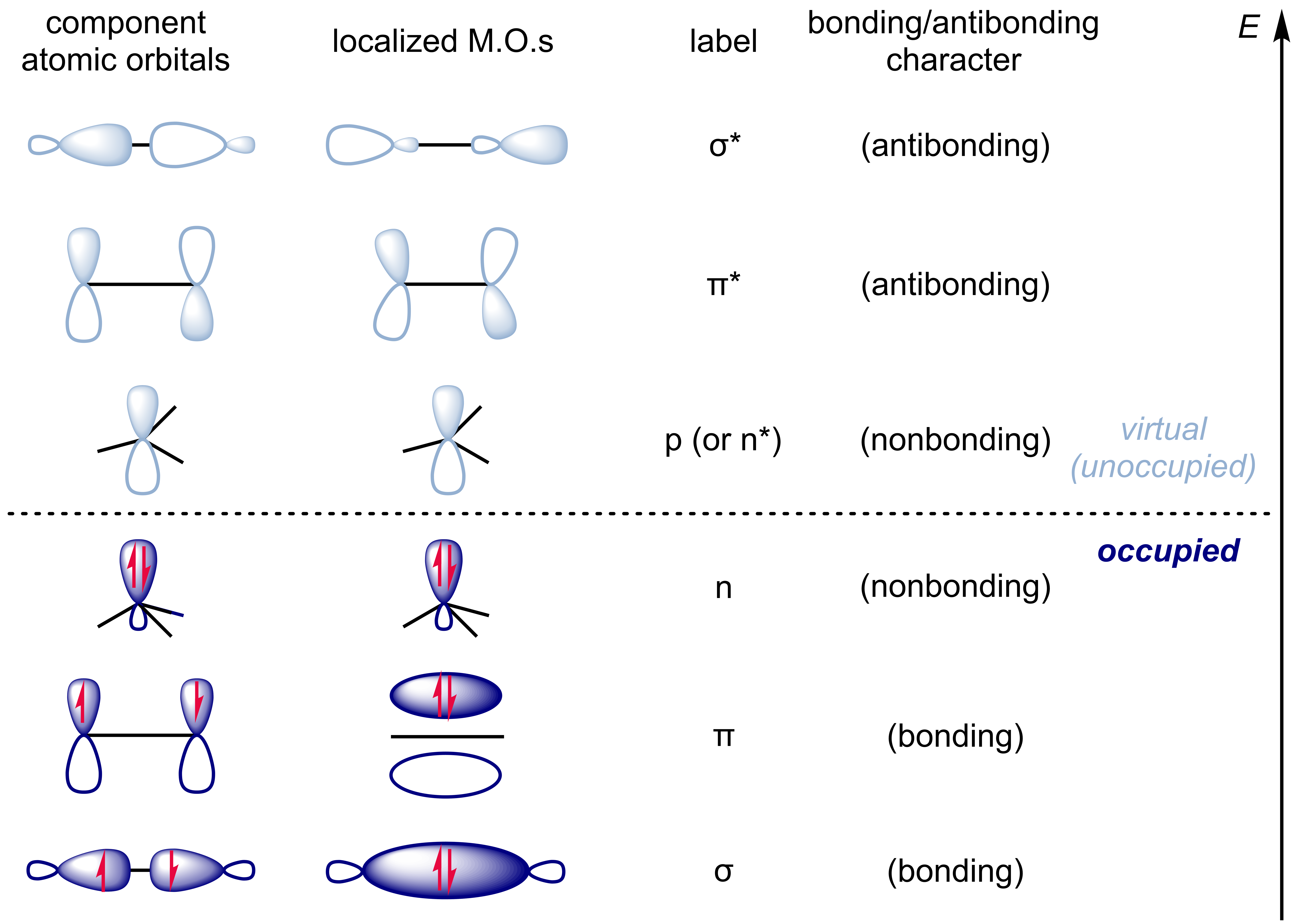
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The local ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |