|
Ionic Transfer
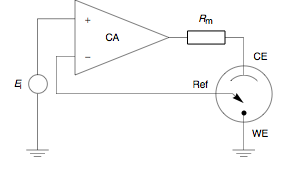
Ionic transfer is the transfer of ions from one liquid phase to another. This is related to the phase transfer catalysts which are a special type of liquid-liquid extraction which is used in synthetic chemistry. For instance nitrate anions can be transferred between water and nitrobenzene. One way to observe this is to use a cyclic voltammetry experiment where the liquid-liquid interface is the working electrode. This can be done by placing secondary electrodes in each phase and close to interface each phase has a reference electrode. One phase is attached to a potentiostat which is set to zero volts, while the other |
Phase Transfer Catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homogeneous catalysis or heterogeneous catalysis methods depending on the catalyst used. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst. PTC is widely exploited industrially.Marc Halpern "Phase-Transfer Catalysis" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Polyesters for example are prepared from acyl chlorides and bisphenol-A. Phosphothioate-based pesticides are generated by PTC-catalyzed alkylation of phosphothioates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the '' reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of Resonance (chemistry), resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents. As confirmed by X-ray crystallography, nitrobenzene is a planar molecule. Production Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (Δ''H'' = −117 kJ/mol). World capacity for nitrobenzene in 1985 was about 1,700,000 tonnes. The nitration process involves formation of the nitronium ion (NO2+), followed by an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Voltammetry
In electrochemistry, cyclic voltammetry (CV) is a type of voltammetric measurement where the potential of the working electrode is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles in potential are repeated until the voltammetric trace reaches a cyclic steady state. The current at the working electrode is plotted versus the voltage at the working electrode to yield the cyclic voltammogram (see Figure 1). Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode. Experimental method In cyclic voltammetry (CV), the electrode potential is ramped linearly versus time in cyclical phases. The rate of voltage change over time during each of these phases is known as the scan rate (V/s). In a standar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reference Electrode
A reference electrode is an electrode that has a stable and well-known electrode potential. The overall chemical reaction taking place in a cell is made up of two independent half-reactions, which describe chemical changes at the two electrodes. To focus on the reaction at the working electrode, the reference electrode is standardized with constant (buffered or saturated) concentrations of each participant of the redox reaction. There are many ways reference electrodes are used. The simplest is when the reference electrode is used as a half-cell to build an electrochemical cell. This allows the potential of the other half cell to be determined. An accurate and practical method to measure an electrode's potential in isolation ( absolute electrode potential) has yet to be developed. Aqueous reference electrodes Common reference electrodes and potential with respect to the standard hydrogen electrode (SHE): * Standard hydrogen electrode (SHE) (E = 0.000 V) activity of H+ = 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A ''Bipotentiostat'' and ''polypotentiostat'' are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively. The system functions by maintaining the potential of the working electrode at a constant level with respect to the reference electrode by adjusting the current at an auxiliary electrode. The heart of the different potentiostatic electronic circuits is an operational amplifier (op amp). It consists of an electric circuit which is usually described in terms of simple op amps. Primary use This equipment is fundamental to modern electrochemical studies using three electrode systems for investigations of reaction mechanisms related to redox chemistry and other chemical phenomena. The dimensions of the resulting data depend on the experiment. In voltammetry, electric current in amps is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ITIES
In electrochemistry, ITIES (interface between two immiscible electrolyte solutions) is an electrochemical interface that is either polarisable or polarised. An ITIES is polarisable if one can change the Galvani potential difference, or in other words the difference of inner potentials between the two adjacent phases, without noticeably changing the chemical composition of the respective phases (i.e. without noticeable electrochemical reactions taking place at the interface). An ITIES system is polarised if the distribution of the different charges and redox species between the two phases determines the Galvani potential difference. Usually, one electrolyte is an aqueous electrolyte composed of hydrophilic ions such as NaCl dissolved in water and the other electrolyte is a lipophilic salt such as tetrabutylammonium tetraphenylborate dissolved in an organic solvent immiscible with water such as nitrobenzene, or 1,2-dichloroethane. Charge transfer reactions of an ITIES Three major ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |